Ecosystems and Human Well-Being: Volume 1: Current State and Trends: Human Infectious Disease Agents
This is Chapter 14 of the Millenium Ecosystem Assessment report Ecosystems and Human Well-Being: Volume 1: Current State and Trends
Coordinating Lead Authors: Jonathan A. Patz, Ulisses E.C. Confalonieri
Lead Authors: Felix P. Amerasinghe, Kaw Bing Chua, Peter Daszak, Alex D. Hyatt, David Molyneux, Madeleine Thomson, Dr. Laurent Yameogo, Mwelecele-Malecela-Lazaro, Pedro Vasconcelos, Yasmin Rubio-Palis
Contributing Authors: Diarmid Campbell-Lendrum, Thomas Jaenisch, Hassane Mahamat, Clifford Mutero, David Waltner-Toews, Christina Whiteman
Review Editors: Paul Epstein, Andrew Githeko, Jorge Rabinovich, Philip Weinstein
Main Messages
According to the World Health Organization, infectious diseases still account for close to one quarter of the global burden of disease. Major tropical diseases, particularly malaria, meningitis, leishmaniasis, dengue, Japanese encephalitis, African trypanosomiasis, Chagas disease, schistosomiasis, filariasis, and diarrheal diseases still infect millions of people throughout the world (very certain).
The magnitude and direction of altered disease incidence due to ecosystem changes depend on the particular ecosystems, type of land use (Land-use and land-cover change) change, disease-specific transmission dynamics, and the susceptibility of human populations. Anthropogenic drivers that especially affect infectious disease risk include destruction or encroachment into wildlife habitat, particularly through logging and road building; changes in the distribution and availability of surface waters, such as through dam construction, irrigation, or stream diversion; agricultural land use changes, including proliferation of both livestock and crops; deposition of chemical pollutants, including nutrients, fertilizers, and pesticides; uncontrolled urbanization or urban sprawl; climate variability and change; migration and international travel and trade; and either accidental or intentional human introduction of pathogens (medium certainty).
There are inherent trade-offs in many types of ecosystem changes associated with economic development, where the costs of disease emergence or resurgence must be weighed against a project’s benefits to health and well-being. Such trade-offs particularly exist between infectious disease risk and development projects geared to food production, electrical power, and economic gain. To the extent that many of the risk mechanisms are understood, disease prevention or risk reduction can be achieved though strategic environmental management or measures of individual and group protection (high certainty).
Intact ecosystems play an important role in regulating the transmission of many infectious diseases. The reasons for the emergence or reemergence of some diseases are unknown, but the main biological mechanisms that have altered the incidence of many infectious diseases include altered habitat, leading to changes in the number of vector breeding sites or reservoir host distribution; niche invasions or interspecies host transfers; changes in biodiversity (including loss of predator species and changes in host population density); human-induced genetic changes of disease vectors or pathogens (such as mosquito resistance to pesticides or the emergence of antibioticresistant bacteria); and environmental contamination of infectious disease agents (high certainty).
Disease/ecosystem relationships that best illustrate these biological mechanisms include the following examples with high certainty (unless stated otherwise):
- Dams and irrigation canals provide ideal habitat for snails that serve as the intermediate reservoir host species for schistosomiasis; irrigated rice fields increase the extent of mosquito breeding areas, leading to greater transmission of mosquito-borne malaria, lymphatic filariasis, Japanese encephalitis, and Rift Valley fever.
- Deforestation alters malaria risk, depending on the region of the world. Deforestation has increased the risk of malaria in Africa and South America (medium certainty).
- Natural systems with intact structure and characteristics generally resist the introduction of invasive human and animal pathogens brought by human migration and settlement. This seems to be the case for cholera, kala-azar, and schistosomiasis, which have not become established in the Amazonian forest ecosystem (medium certainty).
- Uncontrolled urbanization of forest areas has been associated with mosquito-borne viruses (arboviruses) in the Amazon, and lymphatic filariasis in Africa. Tropical urban areas with poor water supply systems and lack of shelter promote transmission of dengue fever.
- There is evidence that habitat fragmentation, with subsequent biodiversity loss, increases the prevalence of the bacteria that causes Lyme disease in North America in ticks (medium certainty).
- Zoonotic pathogens (complete natural life cycle in animals) are a significant cause of both historical diseases (including HIV and tuberculosis) and newly emerging infectious diseases affecting humans (such as SARS, West Nile virus, and Hendra virus).
- Intensive livestock agriculture that uses subtherapeutic doses of antibiotics has led to the emergence of antibiotic strains of Salmonella, Campylobacter, and Escherichia coli bacteria. Overcrowded and mixed livestock practices, as well as trade in bushmeat, can facilitate interspecies host transfer of disease agents, leading to dangerous novel pathogens, such as SARS and new strains of influenza.
Human contact with natural ecosystems containing foci of infections increases the risk of human infections. Contact zones between systems are frequently sites for the transfer of pathogens and vectors (whenever indirect transmission occurs) to susceptible human populations such as urban-forest borders (malaria and yellow fever) and agricultural-forest boundaries (hemorrhagic fevers, such as hantavirus) (high certainty). The different types and subtypes of systems (natural, cultivated, and urban) may contain a unique set of infectious diseases (such as kala-azar or plague in drylands, dengue fever in urban systems, and cutaneous leishmaniasis in forest systems), but some major diseases are ubiquitous, occurring across many ecosystems (such as malaria and yellow fever) (very certain).
Tropical developing countries are more likely to be affected than richer nations in the future due to their greater exposure to the vectors of infectious disease transmission and environments where they occur. Such populations have a scarcity of resources to respond to and plan environmental modifications associated with economic activities (high certainty). However, international trade and transport leave no country entirely unaffected.
The following diseases (high certainty) are ranked as high priority for their large global burden of disease and their high sensitivity to ecological change:
- malaria across most ecological systems;
- schistosomiasis, lymphatic filariasis, and Japanese encephalitis in cultivated and inland water systems in the tropics;
- dengue fever in tropical urban centers;
- leishmaniasis and Chagas disease in forest and dryland systems;
- meningitis in the Sahel;
- cholera in coastal, freshwater (Freshwater biomes), and urban systems; and
- West Nile virus and Lyme disease in urban and suburban systems of Europe and North America.
14.1 Introduction
This chapter focuses on infectious diseases whose incidence has been shown or is suspected to be related to anthropogenic ecological change. Mechanisms of change occur through a variety of ways, including altered [[habitat]s] or breeding sites for disease vectors or reservoirs, niche invasions, loss of predator species, biodiversity change, host transfer, and changes in (intermediate) host population density.
Infectious diseases stemming from health infrastructural deficiencies, such as poor sanitation and lack of adequate vaccine coverage, as well as those linked to specific sociocultural factors, such as airborne and sexually transmitted diseases, are not covered in this chapter, even though these lead to a large global burden of disease. Readers should refer to Chapter 5 (Ecosystems and Human Well-Being: Volume 1: Current State and Trends: Human Infectious Disease Agents) of this volume and Chapter 12 of Policy Responses for an assessment of noninfectious disease and related health topics.
Ecosystems affect human health in many ways, either directly or indirectly. Many major pharmaceuticals, including aspirin, digitalis, quinine, and tamoxifin, originated from plants. (See Chapter 10.) Intact ecosystems protect against mortality and injuries from floods and mudslides. (See Chapter 16 (Ecosystems and Human Well-Being: Volume 1: Current State and Trends: Human Infectious Disease Agents).) Human health depends on access to food, clean water, clean air, and sanitation. (See also Chapters 8 (Ecosystems and Human Well-Being: Volume 1: Current State and Trends: Human Infectious Disease Agents), 7 (Ecosystems and Human Well-Being: Volume 1: Current State and Trends: Human Infectious Disease Agents), 13 (Ecosystems and Human Well-Being: Volume 1: Current State and Trends: Human Infectious Disease Agents), and 27 (Ecosystems and Human Well-Being: Volume 1: Current State and Trends: Human Infectious Disease Agents).) Watershed conditions influence water quality and wetlands help remove toxins from water— increased soil runoff following deforestation, for example, has led to mercury contamination of Amazonian fish. (See Chapter 20.) Toxic algal blooms threaten food safety. (See Chapter 19 (Ecosystems and Human Well-Being: Volume 1: Current State and Trends: Human Infectious Disease Agents)). Watershed protection has been used to offset the cost of drinking water treatment facilities. (See MA, Policy Responses, Chapter 7.) Finally, a broad range of noninfectious disease health risks and prevention strategies are detailed in Chapter 16 of Policy Responses.
Infectious diseases account for 29 of the 96 major causes of human morbidity and mortality listed by the World Health Organization, representing 24% of the global burden of disease (WHO 2004). As numerous reports address the health effects of poor sanitation and drinking water treatment, this chapter focuses more specifically on diseases with known links to anthropogenic ecological change. Table 14.1 and Figure 14.1 (in Appendix A (Ecosystems and Human Well-Being: Volume 1: Current State and Trends: Human Infectious Disease Agents)) show the current extent and distribution of infectious and parasitic diseases around the globe.
| Table 14.1. Burden of Infectious and Parasitic Disease in 2003, by WHO Region and Mortality Stratum. Mortality stratum is a way of dividing up the WHO regions, which are based on geography, into units which are more similar in terms of health performance (i.e., separating Australia, Japan, and New Zealand out from China, the Philippines, and others in the Western Pacific Region, and Canada, the United States, and Cuba from the rest of the Americas, where health status is poorer). They are based on WHO estimates of adult and child mortality, with some arbitrary threshold to group them into different classes (see second column in the table). The data are based on nationally reported health statistics, although there is sometimes some estimation by WHO if national statistics are poor or non-existent. (WHO 2004) | ||||
| Region | Mortality Stratum | Total DALYsa | DALYs from Infectious and Parasitic Diseases |
Infectious and Parasitic Diseases as Share of Total |
| (thousand) | (thousand) | (percent) | ||
| Africa | high child, high adult | 160,415 | 75,966 | 47.4 |
| high child, very high adult | 200,961 | 111,483 | ||
| Americas | very low child, very low adult | 46,868 | 1,228 | 2.6 |
| low child, low adult | 81,589 | 6,719 | 8.2 | |
| high child, high adult | 17,130 | 3,944 | 23.0 | |
| Southeast Asia | low child, very low adult | 62,463 | 10,598 | 17.0 |
| high child, high adult | 365,110 | 78,355 | 21.5 | |
| Europe | very low child, very low adult | 51,725 | 891 | 1.7 |
| low child, low adult | 37,697 | 2,040 | 5.4 | |
| low child, high adult | 60,900 | 2,734 | 4.5 | |
| Eastern Mediterranean | low child, low adult | 24,074 | 1,529 | 6.4 |
| high child, high adult | 115,005 | 30,881 | 26.9 | |
| Western Pacific | very low child, very low adult | 16,384 | 322 | 2.0 |
| low child, low adult | 248,495 | 23,349 | 9.4 | |
| Total | 1,487,816 | 350,039 | 23.5 | |
| a Disability-adjusted life year: years of healthy life lost, a measure of disease burden for the gap between actual health of a population compared with an ideal situation where everyone lives in full health into old age. (WHO World Health Report 2004) | ||||
The incidence of many of these diseases is not declining. According to WHO (WHO 2002), African trypanosomiasis, dengue, and leishmaniasis are emerging and expanding and do not yet have a standardized control program in place. In addition, malaria, schistosomiasis, and tuberculosis persist even though active control programs have been established. Comparison of disease-burden figures published in WHO’s latest World Health Report (2004) with the same statistics from the previous report (WHO 2002) shows that malaria, meningitis, leishmaniasis, dengue, and Japanese encephalitis are increasing. Tropical diseases with essentially no change include diarrheal diseases, trypanosomiasis, Chagas disease, schistosomiasis, and filariasis. However, onchocerciasis (river blindness) shows a declining trend.
While this chapter summarizes known links between ecological degradation and altered infectious disease transmission or emergence, natural systems can also be a source of pathogens, and destruction of an ecosystem may, in some cases, reduce the prevalence of disease in an area. Destroyed ecosystems have led to the disappearance of foci of disease, but this has resulted more from economic development rather than from any planned disease control. Yet environmental modification has been, for millennia, a key means for controlling disease vectors—from the drainage of swamps in Rome to reduce mosquitoes to deforestation in Zimbabwe to protect cattle from trypanosomiasis. At this point in history, however, the scale of ecological change may be leading to disease emergence or reemergence, and this is the issue to which the assessment in this chapter is directed.
14.1.1 Historical Perspective on Infectious Diseases and Development
Over the millennia, people have used and changed the habitable environment. Ten thousand years ago, agriculture and large settlements developed. Several of today’s most pervasive diseases originally stemmed from domestication of livestock. Tuberculosis, measles, and smallpox, for example, emerged following the domestication of wild cattle. Infectious agents or pathogens of vertebrate mammals that infect humans as incidental hosts are called zoonotic, and the resultant diseases are zoonoses. Many pathogens that are currently passed from person to person (anthroponotic), including some influenza viruses and HIV, were formerly zoonotic but have diverged genetically from their ancestors that occurred in animal hosts. Many diseases thought to be caused by noninfectious agents, including genetically based and chronic diseases, are now known to be influenced or directly caused by infectious agents (UNEP in press).
In the last two centuries, the spread of industrial and postindustrial change, rapid population growth, and population movements have quickened the pace and extensiveness of ecological change. New diseases have emerged even as some pathogens that have been around for a long time are eradicated or rendered insignificant, such as smallpox. Environmental and ecological change, pollutants, the widespread loss of top predators, persistent economic and social crises, and international travel that drives a great movement of potential hosts have progressively altered disease ecology, affecting pathogens across a wide taxonomic range of animals and plants (Epstein 1995).
14.1.2 Ecology of Infectious Diseases
Intact ecosystems maintain a diversity (Species diversity) of species in equilibrium and can often provide a disease-regulating effect if any of these species are either directly or indirectly involved in the life cycle of an infectious disease and occupy an ecological niche that prevents the invasion of a species involved in infectious disease transmission or maintenance. Disease agents with much of their life cycle occurring external to the human host, such as water- and vector-borne diseases, are subjected to environmental conditions, and it is these diseases for which most linkages to ecosystem conditions have been found (Patz et al. 2000).
Infectious diseases are a product of the pathogen, vector, host, and environment. Thus, understanding the nature of epidemic and endemic diseases and emerging pathogens is essentially a study of the population biology of these three types of organisms, as well as of environmental factors. In addition to ecologically mediated influences on disease, changes in the level of infectious diseases can themselves disrupt ecosystems (such as bird populations or predator-prey relationships altered by West Nile virus) (Daszak et al. 2000; Epstein et al. 2003).
Recent interest in infectious disease threats to public health has focused on emerging and reemerging pathogens. From a scientific perspective, looking at emerging infectious diseases is useful, as they display different adaptive mechanisms of evolution that have been ‘‘successful’’ in leading to the survival or even increased spread of a microorganism. In a narrow sense, the study of the ecology of emerging infectious diseases tries to understand (and possibly also predict) the mechanisms that lead to the ability to switch hosts and establish in a new host—from the perspective of a given pathogen (as described later in this chapter).
Definitions of the term ‘‘emerging’’ are given early in the literature (e.g., Krause 1981; Lederberg et al. 1992). Emerging diseases are those that have recently increased in incidence, impact, or geographic or host range (Lyme disease, tuberculosis, West Nile virus, and Nipah virus, respectively); that are caused by pathogens that have recently evolved (such as new strains of influenza virus, SARS, or drug-resistant strains of malaria); that are newly discovered (Hendra virus or Ebola virus); or that have recently changed their clinical presentation (hantavirus pulmonary syndrome, for instance). Many authors vary in their definitions of ‘‘recent,’’ but most agree that emerging infectious diseases are those that have developed within the last 20–30 years (Lederberg et al. 1992). ‘‘Reemerging’’ diseases are a subclass of emerging diseases that historically occurred at significant levels but that became less significant due to control efforts and only recently increased in incidence again, such as dengue fever and cholera.
14.1.3 Trade-offs
While preservation of natural ecosystems can prevent disease emergence or spread, there are recognized trade-offs between ecological preservation and human disease. Malaria control efforts, for example, which relied heavily on the insecticide DDT, caused enormous damage to wetland systems and beyond. (See MA Policy Responses, Chapter 12.)
Probably the best documented examples of trade-offs involving ecosystem change, development, and disease are associated with water supply projects for agriculture or electrical power. (See Box 14.1.) Dams and irrigation systems were one of the most visible symbols of water resources development and management in the twentieth century. Irrigation systems are estimated to consume 70–80% of the world’s surface freshwater resources and produce roughly 40% of its food crops. (See Chapter 7 (Ecosystems and Human Well-Being: Volume 1: Current State and Trends: Human Infectious Disease Agents).) The pace of irrigation development has increased rapidly over the past half-century, in order to meet the increasing food requirements of human populations. But irrigation and dam construction can also increase transmission of diseases such as schistosomiasis, Japanese encephalitis, and malaria.
|
|
|
From 1930 to 1970, dams were synonymous with economic development, consuming an estimated $3 trillion in global investments but providing food security, power, local employment, and the expansion of physical and social infrastructure such as roads and schools (WCD 2000). Apart from their direct benefits, dams also are recognized to mediate indirect benefits, both economic and social, that are often ignored in quantifications of economic benefits focused on crop production. These include the use of water for horticulture, livestock farming, fisheries, and domestic purposes. Case studies done for the World Commission on Dams show services and benefits ranging from irrigation and electricity generation for domestic and industrial purposes to flood protection, tourism, fisheries, local employment, and water supply. At the household level it is accepted that irrigation projects, and implicitly large irrigation dams, have contributed to greater food security and improved nutrition. The magnitude of the impact at the national level is less clear. In India (one of the largest builders of irrigation dams), for instance, estimates of total food increase attributable to new land brought under irrigation range from 10% to 30% and nutrition levels have improved by 14% over the past 25 years. Over the past 50 years, the country has achieved a marginal per capita increase in food availability and a decrease in the proportion of rural population below the poverty line (people without the capacity to purchase their own food requirements). However, the absolute number of people below the poverty line has increased by approximately 120 million people (WCD 2000). The financial and economic profitability of large dam projects also presents a mixed picture. The WCD’s evaluation of 14 large dam projects showed a shortfall of roughly 5% in the average economic internal rate of return, between the appraisal estimate and the evaluation estimate, with 4 projects falling below the 10% rate of return that is deemed acceptable in a developing-country economic context. The WCD concluded that irrigation dam projects have ‘‘all too often’’ failed to deliver on the economic profitability promised, even when defined narrowly in terms of direct project costs and benefits. However, added to the direct costs are additional costs in terms of adverse economic, social, environmental, and health impacts, such as the loss of thousands of hectares of tropical forests and their associated flora and fauna, the suffering of physically and livelihood displaced communities, the loss of downstream fisheries and agricultural productivity, and impaired health due to water-related diseases. Diseases such as malaria, schistosomiasis, onchocerciasis, lymphatic filariasis, and Japanese encephalitis have at various times scourged humankind in different parts of the world, and together with diarrheal and intestinal diseases caused by microbial agents and helminths they continue to be a serious threat to human health. There is an extensive literature on disease outbreaks or increased endemicity occurring in the aftermath of large-scale water resources development over the past 50–75 years (for example, Surtees 1975; Service 1984, 1989; Gratz 1987; Hunter et al. 1993; Jobin 1999). However, there is a serious lack of comparative burden of disease estimations and economic cost estimations relating to these disease outbreaks or increased endemicity. |
Such trade-offs also have an important temporal aspect. For example, draining wetlands can reduce mosquito breeding sites for immediate benefit, but the wetland services of filtering, detoxifying, or providing species habitat will be lost.
14.1.4 Ecosystem Services Relevant to Human Health
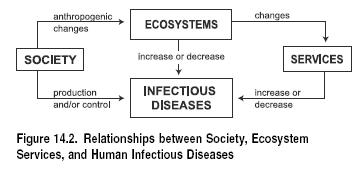
The relationships between ecological systems, their services, human society, and infectious diseases are complex. (See Figure 14.2.) The primary drivers of ecosystem changes are linked to population growth and economic development. These changes trigger several ecological mechanisms that can often increase the risk of infectious disease transmission or can change conditions of vulnerability, such as malnutrition, stress and trauma (in floods and storms, for example), immunosuppression, and respiratory ailments associated with poor air quality.
There is a wide spectrum of human disturbances to ecosystems and their services that may change disease risk via biological mechanisms described in the next section. Of course, human activities not associated with environmental modifications may also have a role in the production of infectious diseases, both in their emergence or resurgence. This is the case of the infectious processes associated with human behavior, such as those transmitted by direct contact (such as AIDS or skin infections), airborne infections, and some food-borne infections.
Ecosystem changes can mediate the influence of anthropogenic activities in changing the epidemiological patterns of human infectious diseases by reducing or increasing disease incidence. (See Box 14.2.) Most ecological systems have a unique set of infectious diseases; however, some diseases, such as malaria, are more ubiquitous and can be found across ecological systems such as drylands, forests, and wetlands, although with somewhat different dynamics.
|
|
|
The Amazon forest system in Brazil has been the subject of successive cycles of occupation and development since the last quarter of the nineteenth century, starting with the rubber boom up to the 1980s, when road building and the expansion of cattle ranching also became important drivers. These developments attracted human migrations from other parts of the country, either for temporary work or permanent settlement (Confalonieri 2001). Most of the migrants came from northeastern Brazil, which is endemic for diseases like kala-azar, schistosomiasis, and Chagas disease. As for the introduction of schistosomiasis, both infected humans and snail intermediate hosts have been found in the region (Sioli 1953). However, the foci of the disease were established only in the periphery of a few major cities, in snail breeding sites created by humans, such as pools, ponds, and channels. No foci of transmission of the disease were created among the riverine populations, some including infected human migrants. The main reason was the absence of the snail species Biomphalaria spp, which were not able to develop probably due to the characteristics of the fresh water of the natural systems, which do not have the appropriate mineral salts necessary for the formation of the shell of the snails (Sioli 1953). A similar situation has been observed with kala-azar, which is endemic in rural areas of the Brazilian northeast, involving humans, sand flies, dogs, and wild canids as reservoir animals. So far the disease has be come established only in two [[geographic]ally] restricted areas of the Brazilian Amazon: in a savanna area in the northern part and in a periurban setting in the central part (Confalonieri 2000). In both situations it seems that the dogs are the only reservoir species involved in the transmission cycle, and the pathogen did not pass to wild populations of vertebrates (Guerra 2004; Silveira et al. 1997). In the early 1990s, cholera entered Brazil through the Peruvian border and moved down the Amazon River and its tributaries, where a few small outbreaks occurred. When the ‘‘cholera wave’’ reached the major cities in the Amazon region, hundreds or even thousands of people were affected. In the ‘‘rural’’/riverine areas of western Brazilian Amazon, the disease affected people only for a few months and vanished without any significant control measures being implemented. The suspected major reasons for this, in addition to the low human population density in the area, was that the left margin tributaries of the Amazon River were unsuitable for the survival of Vibrio cholerae, due especially to their low pH. These case studies are good example of the ‘‘nonreceptiveness’’ of natural systems—and the people living on them—to the introduction of alien pathogens or parasites and their invertebrate carriers, which did not evolve in these environments due to a natural resilience. This has important implications both for public health and for conservation. |
14.2 Trends and Drivers of Changes in Disease Risk
Most emerging diseases are driven by human activities that modify the environment or otherwise spread pathogens into new ecological niches (Taylor et al. 2001). Examples of direct anthropogenic drivers that affect disease risk include wildlife habitat destruction, conversion, or encroachment, particularly through deforestation and reforestation; changes in the distribution and availability of surface waters, such as through dam construction, irrigation, and stream diversion; agricultural [[land use (Land-use and land-cover change) change]s], including proliferation of both livestock and crops; deposition of chemical pollutants, including nutrients, fertilizers, and pesticides; uncontrolled urbanization; urban sprawl; climate variability and change; migration and international travel and trade; and either accidental or intentional human introduction of pathogens. (See also Chapter 3.)
These anthropogenic drivers of ecosystem disturbance can lead to specific changes in ecosystems that may or may not lead to disease emergence via mechanisms that are more directly relevant to life cycles or transmission of infectious diseases. There is concern that the extent of ecosystem changes in recent decades and the multiple ways in which [[habitat]s] and biodiversity are being altered are increasing the odds that infectious diseases will be affected at some level. The specific biological mechanisms altering disease incidence, emergence, or reemergence are described here and, by way of illustration, disease case studies in this chapter are organized according to these biological mechanisms.
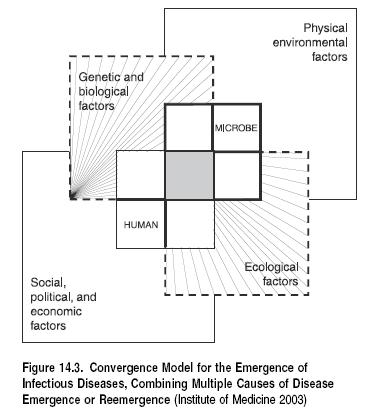
The relationship between infectious diseases and ecological changes is shown in Figure 14.3.
Disturbance or degradation of ecosystems can have biological effects that are highly relevant to infectious disease transmission. The reasons for the emergence or reemergence of some diseases are unknown, but the following mechanisms have been proposed and have altered the incidence of many diseases (Molyneux 1997; Daszak et al. 2000; Patz et al. 2004):
- altered habitat leading to changes in the number of vector breeding sites or reservoir host distribution;
- niche invasions or transfer of interspecies hosts;
- biodiversity change (including loss of predator species and changes in host population density);
- human-induced genetic changes in disease vectors or pathogens (such as mosquito resistance to pesticides or the emergence of antibiotic-resistant bacteria); and
- environmental contamination by infectious disease agents (such as fecal contamination of source waters).
While the rest of this chapter is organized according to these mechanisms (see Table 14.2), it is important to recognize that in many instances these often overlap or act in combination, sometimes resulting in non-linear or synergistic effects on disease transmission. It should be noted that emerging or resurging diseases may occur across many ecosystems even though they have been grouped here according to the major ecosystem in which they are prevalent.
| Table 14.2. Mechanisms of Disease Emergence and Examples of Diseases across Ecosystems | |||||
| Mechanisms | Ecosystems | ||||
| Cultivated Systems | Dryland Systems | Forest Systems | Urban Systems | Coastal Systems | |
| Habitat alteration |
schistomiasis Japanese encephalitis meningitis |
hantavirus Rift Valley fever Rift Valley fever |
malaria arboviruses (e.g., yellow fever) onchocerciasis |
lymphatic filariasis Dengue fever malaria |
cholera |
| Niche invasion or host transfer |
Nipah virus BSE (mad cow) SARS influenza |
HIV (initially) | leishmaniasis | ||
| Biodiversity change |
leishmaniasis | onchocerciasis | rabies onchocerciasis |
lyme disease | |
| Human-driven genetic changes |
antibiotic-resistant bacteria | chagas disease | chagas disease | ||
| Environmental contamination of infections agents |
cryptosporidiosis leptospriosis |
leptospriosis | diarrheal diseases | ||
14.2.1 Altered Habitat/Breeding Sites: Effect on Infectious Disease Transmission
Disturbance of habitats due to alterations in land cover (Land-use and land-cover change) or climatic change is considered to be the largest factor altering the risk of infectious diseases—for example, by affecting breeding sites of disease vectors or the biodiversity of vectors or reservoir hosts. Examples of diseases emerging or resurging due to habitat change that occur across many ecosystems are described here for cultivated, drylands, forest, urban, and coastal systems.
14.2.1.1 Irrigation and Water Development in Cultivated Systems
According to the FAOSTAT database (apps.fao.org/default.jsp), the global extent of irrigated agricultural land increased from 138 million hectares in 1961 to 271 million hectares in 2000. In 1950, there were an estimated 5,000 large irrigation and multipurpose dams in the world, which has now increased to more than 45,000. These provide water for 30–40% of irrigated agricultural land and generate 19% of global electricity supplies. (See Chapter 8 (Ecosystems and Human Well-Being: Volume 1: Current State and Trends: Human Infectious Disease Agents) for more on water impoundment and offtake for irrigation.)
Inevitably, this growing trend in water resources development has resulted in qualitative and quantitative changes in natural biodiversity and in changed levels of interaction between humans, vectors, and disease agents. Large-scale human resettlement, which usually occurs in conjunction with irrigation development, has resulted in exposure to disease in nonimmune populations. Furthermore, multiple cropping has changed movement patterns of temporary agricultural labor, increasing risks of disease transmission and dissemination, while overcrowding and low nutritional status has increased susceptibility to infection. Water resources development has often been accompanied by invasions of new disease-carrying vectors or population changes in existing vectors, as well as similar changes in disease agents, which have increased the risks of disease (Bradley and Narayan 1988).
Global statistics on major microbial and parasitic diseases associated with water resource development show differential degrees of mortality and morbidity due to different diseases. Comparisons in terms of disability-adjusted life years (DALYs) show that infectious and parasitic diseases contribute 23.5% (340 million DALYs) of the total global burden of disease.
Unfortunately, DALY estimates only exist for global and regional scales at present, and it is not possible to use these to make direct spatial or temporal comparisons of disease burdens associated with large-scale water resources developments. Such information would be needed to evaluate properly the impact of water resource developments on human health. A similar caveat applies to economic indicators that point to significant impacts. For instance, malaria alone is estimated to reduce the economic growth rate in seriously affected countries by 1.3% per year and to cost these countries billions of dollars a year (Malaney et al. 2004). Once again, however, such figures cannot be disaggregated to determine the economic costs associated with water resources development in particular.
Small dams, below 15 meters in height, are far more numerous than large dams and are closely linked to agriculture. They have been advocated by the international financing community as manageable and practical solutions to land and water conservation. They serve more purposes than large dams: for example, a small multipurpose project may provide water for domestic supplies, fishing, cattle, and irrigation while providing flood control. Although there is no accurate estimate of the number of small dams in the world, it is likely that their collective volume is greater than that of large dams. In Nigeria and Zimbabwe, for instance, the shore length of small dams has been estimated to be 8–10 times that of the large reservoirs (Jewsbury and Imevbore 1988).
Similarly, it is estimated that small dams have an equal or greater impact on human health than large dams. There is usually a high degree of water contact with people and animals, so disease transmission rates are high (Hunter et al. 1993). However, there have been relatively few epidemiological studies on disease trends around small dams in tropical countries, despite available data that show a strong association between small dams and substantial increase in disease. For instance, intense transmission of diseases such as schistosomiasis, onchocerciasis, malaria, lymphatic filariasis, and dracunculosis are associated with small dams in many African countries, including Cameroon, Kenya, Ghana, Mali, Rwanda, and Zambia (Hunter et al. 1993). As in the case of large water resource developments, comparative health statistics in terms of DALYs or other useful parameters such as years of life lost or years lived with disability are yet to be determined.
14.2.1.1.1 Schistosomiasis
Irrigation canals are known to provide ideal habitat for the snails that serve as an intermediate reservoir host for schistosomiasis. For example, reduced salinity and increased alkalinity of water associated with irrigation development along the Senegal River have been shown to increase fecundity and growth of freshwater snails (Southgate 1997).
A literature review of the association between schistosomiasis and the development of irrigation projects along the Tana River in Kenya has been provided by Mutero (2002). Two species of the genus Schistosoma occur in Africa, namely S. haematobium and S. mansoni. Clinical signs for the two infections are blood in the urine and blood in the stools, respectively. S. haematobium has the highest prevalence along the lower Tana, where the now largely abandoned Hola irrigation scheme is situated. In 1956, when the scheme began, there were no snail vectors of S. haematobium due to a lack of suitable habitat because of the scheme’s elevation above the river. A decade later, there was a 70% prevalence of urinary schistosomiasis among local children, which rose to 90% by 1982 due to poorly maintained irrigation channels.
14.2.1.1.2 Mosquito-borne diseases and tropical rice irrigation
There are many examples worldwide of vector-borne disease problems linked to water resources development (see reviews of Bradley 1977; Mather and That 1984; Service 1984). (See Box 14.3.) Tropical rice irrigation systems, in particular, have been linked to vector-borne diseases such as malaria and Japanese encephalitis (reviews by Lacey and Lacey 1990; Amerasinghe 2003). Major ecological impacts of irrigated rice include an enormous increase in the extent of mosquito breeding surface and an increase in the availability of habitat where multiple cropping occurs. These factors may selectively favor some species and displace or change the relative dominance of certain species or genotypes.
|
|
|
Two of the most recently documented examples of ecosystem disturbance and mosquito-borne disease are from South Asia, and they provide contrasting examples of the aggravation of health problems resulting from irrigation development in a tropical environment (Sri Lanka) and a more northern desert region (India). In Sri Lanka, the Accelerated Mahaweli Development Project developed 165,000 hectares of land (much of it forested land previously unoccupied by humans) for irrigated rice cultivation, resulting in the settlement of 1 million persons into the malaria-endemic lowland dry zone of the country between 1980 and 1990. Varied impacts of mosquito-borne diseases were observed in the irrigated rice systems. Malaria increased two- to fivefold in all systems within the first two to three years of settlement (Samarasinghe 1986). Plasmodium falciparum infections increased from the normal 5% of infections to 24% in some regions. Some systems have remained highly malarious 10–15 years later, but the disease burden has decreased in other areas. Upstream impacts also were recorded, with outbreaks of malaria in villages along the banks of the Mahaweli River in normally non-malarious hill country areas—a consequence of decreased water flow, pooling, and vector breeding as a result of water impoundment at upstream dams (Wijesundera 1988). The major vector of malaria in Sri Lanka is Anopheles culicifacies, but in areas of the Mahaweli Project it was observed that additional species such as An. annularis and An. subpictus also played a significant role in transmission, as their populations increased due to the availability of suitable breeding habitats (Amerasinghe et al. 1992; Ramasamy et al. 1992). On top of the malaria burden came Sri Lanka’s first major epidemic of Japanese encephalitis in System-H of the Mahaweli in 1985–86 (more than 400 cases and 76 deaths), followed by a second epidemic in 1987–88 (more than 760 cases and 138 deaths). The catalyst appears to have been the promotion of smallholder pig husbandry in a misguided attempt to generate supplementary income among farmers. In a rice irrigation system where Culex tritaeniorhynchus and other Culex vectors of Japanese encephalitis were breeding prolifically, the outcome was catastrophic. The Mahaweli represents a complex of gross physical ecosystem disturbance in terms of forest clearing, dam, reservoir and canal construction, and the maintenance of standing or flowing water virtually throughout the year, which erased the normal trend of wet and dry periods. Added to this was biological disturbance in terms of the replacement of a diverse natural forest flora and fauna by the introduction of a virtual crop monoculture (rice), and a dominant large mammal population (humans, often from areas nonendemic to diseases such as malaria and Japanese encephalitis), together with fellow-traveler species (garden plants, vegetables, fruit trees, livestock, domestic pets, poultry, rodents, and so on). Whether overall plant and animal biodiversity was diminished or not is debatable, but it is clear that the natural biodiversity was replaced by a crop-related one that afforded opportunities for disease causing–organisms and their vectors to have an impact. The development of irrigated agriculture in the Thar Desert, Rajasthan, in northwestern India provides another telling example of ecosystem disturbance exacerbating disease burden. Here, the major change was the provision of surface water to a desert area. The Thar Desert was traditionally only mildly prone to malaria, but in the last six decades it has undergone drastic change in physiography and microclimate concomitant with irrigation development. Contrasting trends in the balance between An. culicifacies (an efficient vector) and An. stephensi (a poor vector) have occurred in the Thar Desert, with An. stephensi constituting 94% of the two species in desert areas, but the opposite situation (overwhelmingly more An. culicifacies) holding in the irrigated areas. As a result, the prevalence of malaria in the irrigated areas has increased almost fourfold between the 1960s and today, with several epidemics in the past 15 years. As in Sri Lanka, this has been accompanied by a high incidence of P. falciparum infections in the irrigated areas, rising from 12% in 1986 to 63% by 1994 (Tyagi 2002). Although excessive rainfall triggered by the El Niño Southern/Oscillation has probably contributed to malaria epidemics in the Thar Desert, Tyagi (2002) relates most of the recent epidemics to the phenomenon of ‘‘inundative vectorism’’—the sudden ushering of one or more vector species in prodigiously high densities in virgin areas such as a recently irrigated desert). Malaria in the Thar Desert is now effectively transmitted in three ways: in the irrigated area it is transmitted in tandem by the native An. stephensi and invader An. culicifacies; in the dryland areas, by An. stephensi; and in the non-command flood-prone southern areas, mainly by An. culicifacies. |
With malaria, in particular, the result can be marked changes in disease equilibrium, which may increase or decrease depending on the transmission capability of a particular mosquito species (Akogbeto 2000). The varied epidemiology of malaria in different cultivated systems in Africa is aptly reviewed by Ijumba and Lindsay (2001), who coined the phrase ‘‘paddies paradox’’ to describe situations where irrigation increases vector populations but may or may not increase malaria.
This anomaly has been largely attributed to differences in socioeconomic and ecological environments inside and outside irrigation schemes. For instance, villages surrounded by irrigated rice fields in Kenya showed a 30- to 300-fold increase in the number of the local malaria vector, Anopheles arabiensis, compared with those without rice irrigation, yet malaria prevalence was significantly lower in these villages (0–9% versus 17–54%) (Mutero et al. 2003). The most plausible explanation for this appeared to be the tendency of An. arabiensis to feed more on cattle than people in irrigated villages.
In rural India during the 1990s, ‘‘irrigation malaria’’ was responsible for endemic transmission in a population of about 200 million people (according to Sharma 1996). This has been attributed to poorly maintained irrigation systems, illegal irrigation, water seepages, poor drainage, and a rise in water tables associated with irrigation that created conditions suitable for the breeding of the major vector An. culicifacies and slow running streams that favor another vector, An. fluviatilis.
Japanese encephalitis is confined to Asia and is almost always associated with rice ecosystems. Region-wide, with an estimated 50,000 cases per year and 20% fatality and disability rates, the disease takes a considerable social and economic toll (Hoke and Gingrich 1994). The primary vector, Culex tritaeniorhynchus, occurs throughout Asia and breeds abundantly in flooded rice fields, as does another important vector, C. vishnui (India, Thailand, Taiwan). Other vectors, such as C. gelidus (in Indonesia, Sri Lanka, Thailand, and Viet Nam), C. fuscocephala (in Malaysia, Thailand, Taiwan, and Sri Lanka), and C. annulus (in Taiwan), breed in a variety of [[habitat]s], some of them associated with irrigated rice.
The transmission cycle of Japanese encephalitis involves an amplifying host, which is usually the domestic pig (in some instances, birds of the heron family (Ardeidae) are also involved) (Hoke and Gingrich 1994). Thus, irrigated riceland communities in which pig husbandry is traditionally carried out are likely sites for the disease, as both vector and amplifying host are brought together. The disease is endemic in irrigated ricelands in Thailand and China. Explosive outbreaks of Japanese encephalitis in new irrigation systems have been reported from the Terai region of Nepal and in Sri Lanka (Joshi 1986; Peiris et al. 1992). Extensive use of synthetic nitrogenous fertilizers in Indian rice fields has been blamed for significant increases in the populations of this disease’s vectors; elevated nitrogen in the rice field water increases the density of mosquito larvae (Victor and Reuben 2000; Sunish and Reuben 2001).
Another disease linked to irrigation developments is lymphatic filariasis. Commonly called ‘‘elephantiasis,’’ filariasis is caused by a mosquito-borne helminth. Outbreaks often occur from rising water tables following water project developments. In Ghana, for instance, rates of infection, worm load, annual bites per person, and annual transmission potential have been found to be higher in irrigated areas than in communities without irrigation (Appawu et al. 2001). This was also confirmed by another observation, where opening irrigation channels during the dry season resulted in a significant increase of filariasis vectors (Dzodzomenyo et al. 1999). On the other hand, conversion of swamps into rice fields on Java Island, Indonesia, resulted in a decrease of breeding sites for vectors and therefore a decrease in disease transmission (Oemijati et al. 1978).
14.2.1.1.3 African trypanosomiasis (African sleeping sickness)
Tsetse flies are widely distributed in West and Central Africa and parts of East Africa; they are the vectors of animal and human trypanosomes that cause African sleeping sickness. They are a highly adaptable group of generalized vectors feeding on available hosts throughout their distribution and are associated with the ability to adapt rapidly to changing habitats and vegetation. For instance, in Kenya in 1964 an outbreak of sleeping sickness was associated with the spread of flies from the natural shoreline habitats of Lake Victoria to vegetation within settlements characterized by thickets of Lantana camara; flies were feeding on humans and cattle, with cattle acting as the reservoir host of the parasite. More recently, during the 1980s an epidemic in Busoga, Uganda, was the result of civil unrest and abandonment of traditional agricultural practices and crops (coffee and cotton), followed by the spread of L. camara along village edges (UNEP in press).
In West Africa the behavior of tsetse flies in Southeast Nigeria and Côte d’Ivoire peridomestic populations has been closely associated with villages with a high population of domestic pigs and, again, Lantana as well as other vegetation (coconuts, yams, and bananas). In West Africa, Glossina palpalis and G. tachinoides appear to feed preferentially on pigs, where they act as a ‘‘dilution host’’—reducing the risk of sleeping sickness to humans, as do cattle in Uganda (UNEP in press).
14.2.1.1.4 Rodent-borne hemorrhagic viruses
These infections are caused by different species of arenaviruses, with wild rodents of the genera Calomys, Sigmodon, Akodon, and Zygodontomys acting as their natural hosts and reservoirs. They have been especially recorded in Argentina (Junin virus), Bolivia (Machupo virus), and Venezuela (Guanarito virus) (Simpson 1978; Salas et al. 1991; Maiztegui 1975; de Manzione et al. 1998).
These infections often occur in outbreaks involving a few dozen to thousands of cases, mainly in rural populations, and humans become infected through contact with the urine and feces of infected rodents. In the specific case of Junin virus infections there is an additional occupational component: agricultural workers risk excess exposure during the harvesting of corn.
Human infection of the three diseases occurs in both villages and the wider countryside, primarily due to the contact between susceptible human hosts and the naturally infected rodent species in agro-ecosystems. In short, these viral infections are linked to the expansion of agriculture into natural systems in South America.
14.2.1.2 Habitat Change and Disease in Drylands and Grasslands
As with other systems, drylands have specific components and services that are relevant to human health issues. (See Box 14.4.) These can be grouped in two general categories: those that are part of natural systems (either biological or nonbiological) that can pose risks to human health and those associated with social interventions to promote human livelihoods in dry environments.
|
|
|
No animal reservoirs of infections are involved in the transmission of meningococcal meningitis in Africa. This is an airborne disease, transmitted from person to person through aerosols that are inhaled. The disease occurs in high endemic levels in Africa only in the so called meningitis belt, which is a large dry area in the Sahel. The specific environmental mechanisms involved in determining the biological vulnerability of the human population to this infection is not well known, but it is generally assumed that the low relative humidity is an important factor in decreasing the resistance of the upper respiratory tract. The role of dust storms is also being investigated (Molesworth et al. 2002). The countries of Sahelian Africa sandwiched between the hot dry Saharan Desert and the moist humid forests of the Guinea Coast are among the poorest in the world. The region includes significant portions of Senegal, Mauritania, Mali, Burkina Faso, Niger, Chad, Sudan, and Eritrea, which roughly correspond to the zone receiving between 200 and 600 millimeters of annual rainfall. In this region, approximately 90% of the population depends on subsistence agriculture. As a result of rapid demographic change, climate variability, and economic drivers, farmers have expanded their cropping areas to increase production. Agricultural intensification (often resulting in overgrazing) coupled with higher population densities in many areas has led to fallow periods that are insufficient to recuperate the soil. This has resulted in the loss of topsoil due to wind erosion, thus increasing the sources of atmospheric dust. Wind erosion may cause three types of agricultural damage: sedimentation at undesired places, crop damage, and soil degradation (Stark 2003). Wind erosion has resulted in an increase in local dust storms, widely considered to be related to ill health during the dry season. For instance, in a detailed study of farmers’ perceptions on the causes and consequences of wind erosion in Niger, its impact on health (fever, coughing, and sore eyes) was of greater concern than its contribution to crop dam age or loss of topsoil (Bielders 2001). Dramatic increases in atmospheric dust in Sahelian West Africa have been noted in recent years (Ben Mo- hamed 1986; N’tchayi 1994; Nicholson 1998); atmospheric dust emanating from this region and the Sahara has been implicated in respiratory problems many thousands of miles away (Prospero 2001). Dust storms have also been implicated in changes in the spatial and temporal dynamics of meningococcal meningitis epidemics in the region (Molesworth 2002). Key factors that have been identified as determinants of areas at risk of epidemic meningitis are land cover and absolute humidity (Molesworth 2003). The identification of these determinants has significant implications for directing essential monitoring and intervention activities and health policy. It also provides a basis for monitoring the impact of climate variability and environmental change on epidemic occurrence in Africa. |
Water availability is the major limiting factor in drylands—not only for the survival of wild species and for agricultural and livestock production systems (see Chapter 22 (Ecosystems and Human Well-Being: Volume 1: Current State and Trends: Human Infectious Disease Agents)), but also for human health. Water scarcity and the poor quality of available water can increase the risk of transmission of pathogens associated with poor hygiene practices, leading to food-borne and water-borne diseases, which are major health problems in impoverished communities living in these areas. The greatest impacts are most often experienced by the more vulnerable social segments, such as high morbidity and mortality rates in children due to diarrheas. These infections are aggravated by the already existing chronic physical health problems common in impoverished communities, such as malnutrition, as well as a lack of adequate medical care in what are often marginalized communities.
Climate extremes such as droughts can have severe impacts on human health via different pathways, both direct and indirect. Direct effects are basically associated with the exacerbation of water scarcity as well as with food deprivation, resulting in famines. Droughts also have indirect effects that are mediated by social and demographic mechanisms such as individual and population stress and migrations. Movements of rural communities deprived of water and food in extreme situations can become an important determinant of the spatial redistribution of endemic infectious diseases. This is the case of kala-azar in northeastern Brazil. In the major droughts of the early 1980s and 1990s, massive intraregional migrations of people from endemic rural areas to cities in search of subsistence and governmental assistance created new foci of the disease. This has resulted in outbreaks of infectious diseases at the periphery of major cities (Confalonieri 2001).
Besides natural biotic and abiotic factors, anthropogenic environmental modifications in dryland areas can also create conditions for the emergence of health problems related to development projects in these areas. This is the case of the efforts to increase food production in drylands by designing schemes for the provision of water, such as irrigation, and the building of dams has resulted in an increase in the incidence of tropical diseases, as mentioned earlier.
Wild fauna and insect ecology is also important for some infectious diseases located in dryland systems, including plague in the scrublands of southwestern United States, northeastern Brazil, and Peru and in regions of India and southern Africa. Another focal infection is kala-azar (Leishmania donovani), which is present mostly in arid areas such as Sudan (Thomson et al. 1999), Mediterranean countries, and South America.
14.2.1.2.1 Hantavirus
Rodent-borne hantavirus occurs both in arid grasslands (for example, in North America), as well as agricultural systems (particularly in South America and Asia). One of the best-known outbreaks occurred in the spring and summer of 1993, when acute respiratory distress with a high fatality rate was diagnosed among previously healthy individuals in the Four Corners region of the southwestern United States (Engelthaler et al. 1999). The disease, hantavirus pulmonary syndrome (HPS), was traced to infection by a previously unrecognized hantavirus. The virus (Sin Nombre virus) was found to be maintained and transmitted primarily within populations of a common native field rodent, the deer mouse Peromyscus spp. Transmission to humans is thought to occur through contact with virus in secretions and excretions of infected mice.
Recent studies have now shown that the El Ni ño effects during 1991–92 helped boost the reservoir populations of rodents in the region (Engelthaler et al. 1999; Glass et al. 2000). Unseasonal rains during the usually dry summer months in 1992 produced favorable environmental conditions in the spring and summer of 1992 that led to the outbreak of HPS. Parmenter and colleagues reported that populations of deer mice at the Long-Term Ecological Research station approximately 90 kilometers south of Albuquerque, New Mexico, were ten- to fifteenfold higher during the HPS outbreak period than the previous 20–year average (Parmenter et al. 1993). Glass and colleagues (2000) further showed the potential of using remotely sensed data to monitor conditions and identify high-risk areas up to a year in advance of anticipated disease outbreaks.
14.2.1.2.2 Rift Valley Fever
Extensive Rift Valley Fever outbreaks were not reported until 1951, when an estimated 20,000 people were infected during an epidemic among cattle and sheep in South Africa. Outbreaks were reported exclusively from sub-Saharan Africa until 1977–78, when 18,000 people were infected and 598 deaths were reported in Egypt (CDC 2002).
All known Rift Valley Fever virus outbreaks in East Africa from 1950 to May 1998, and probably earlier, followed periods of abnormally high rainfall (Woods et al. 2002). Analysis of these records and of Pacific and Indian Ocean sea surface temperature anomalies, coupled with vegetation data from satellites, showed that accurate predictions of Rift Valley Fever outbreaks in East Africa could be made up to five months in advance. Concurrent near-real-time monitoring with such data may identify actual affected areas (Linthicum et al. 1999).
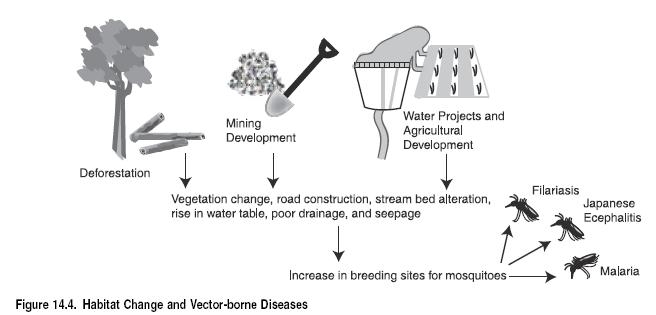
Dams and irrigation can increase the breeding sites of the Rift Valley Fever vector, exacerbating the effect of extreme rainfall. (See Figure 14.4.) Several ecological changes have been reported in the epidemic region in Mauritania following dam construction, irrigation, and heavy rainfall (Jouan et al. 1990). Environmental factors, such as hydroelectric projects, and low-grade transmission among domestic animals could have enhanced the disease’s survival and subsequent outbreaks (Lefevre 1997).
14.2.1.3 Habitat Change and Disease in Forest Systems
The major vector-borne diseases are focused in the tropics. There is a significant overlap between the distribution of the majority of important vectors of human and animal diseases and the biological richness of tropical rain forest ecosystems, woodland savannas, and boundaries of these ecosystems. (See Box 14.5.) It is the degradation of these ecosystems, the behavior and ecology of the vectors at the forest edge, the impact of deforestation on the interactions between humans with vectors, and reservoir hosts at the interface that determine the epidemiology of human infective agents. Additional factors are the behavior and degrees of immunity of local or migrant populations, their interaction with and the behavior of reservoir hosts, and the availability and effectiveness of surveillance systems and quality of local health care (UNEP in press).
|
|
|
Prior to the 1970s, onchocerciasis (also known as river blindness) was a neglected disease. Its devastating effects were largely borne by rural populations of West Africa living near the fast-flowing rivers of the Sahel. When the Onchocerciasis Control Programme was started in 1974, some of West Africa’s richest riparian lands were uninhabited. In villages sited in river valleys near to the white water rapids that form the major breeding sites of the blackfly vector, it was not unusual to find 60% of adults afflicted with the disease and 3–5% blind. As a direct consequence of the disease, communities were forced to abandon their villages en masse. Today, 30 years and $600 million after the program was first launched, the disease has been controlled through one of the most successful public health campaigns in history (Benton 2002). The filarial worm that causes the disease (Onchocerca volvulus) is transmitted in West Africa solely by blackflies, which are members of the Simulium damnsum species complex. Understanding the spatial and temporal distribution of the vectors of onchocerciasis has been key to their successful control (Boakye et al. 1998), given that the S. damnosum species complex comprises many distinct sibling species with varying capacities to transmit O. volvulus. The savanna species S. sirbanum and S. damnosum s.str. were identified early on in the program as the species associated with the blinding form of the disease. The distribution of the different members of the S. damnosum species complex is generally related to vegetation zones, forest, and savanna, but seasonal changes in their distribution occurs on an annual cycle as the monsoon winds and their accompanying rainfall aid dispersal and result in enhanced river flow and the creation of breeding sites. According to Baker (Baker et al. 1990), members of the S. damnosum complex move average distances of 15–20 kilometers daily and may migrate over a total distance of 400–500 kilometers. From the beginning of the control program, vector control has been dogged by ‘‘reinvasion’’—the long-range northwards movement of female blackflies with the potential to reestablish breeding grounds in savanna areas during the wet season and to carry O. volvulus parasite back into vector-cleared areas. Flies are known to be carried from more southerly permanent breeding sites on a generally northeasterly tack across West Africa by the associated winds. In the late 1980s, a reverse form of migration was also noted in which savanna flies, both S. sirbarnum and S. damnosum s.str., appeared to have extended their range into areas previously only inhabited by forest species (Thomson et al. 1996). The migrations of savanna species of S. damnosum s.l. into the forest zones was considered a serious threat to the health of populations living in the forested area, as it suggested that the blinding, savanna form of the disease may spread to the forested areas. In 1988 a Task Force of the Onchocerciasis Control Programme in Sierra Leone determined that breeding sites there were the source of the annual invasion of savanna species of S. damnosum that were actively recolonizing the controlled breeding sites of Mali each rainy season. The Task Force also found that the savanna fly S. sirbarnum was widely distributed throughout the country, including many forested areas (Baker 1989). This has led to the speculation on the possible role of deforestation and rainfall decline on the distribution of different species of S. damnosum s.l. (Walsh et al. 1993), a role that was later confirmed by a detailed cytotaxonomic study of S. damosum larvae found breeding in a deforested area in Ghana (Wilson et al. 2002). Deforestation in West Africa has been implicated in the southward movement of the savanna species of S. damnosum s.l. in the region—the most significant vectors of the blinding form of onchocerciasis (Wilson 2002; Thomson and Connor 1996). This has important implications for the newly developed African Programme for Onchocerciasis Control; unlike its predecessor, the Onchocerciasis Control Programme that successfully controlled savannah species of S. damnosum s.l. using insecticides, the new program is heavily dependant on the widespread distribution of the micro-filaricidal drug ivermectin. Should current control measures fail, then emergence of the blinding disease in deforested areas can be expected. Curiously, forest cover has further implications to the success of new program, as the recent distribution of ivermectin in forested areas has resulted in a number of deaths—thought to be related to the presence of yet another filarial worm, Loa loa. This parasite, which has emerged from obscurity, is now considered a major impediment to the success of the control program, and mapping its potential distribution has become a priority activity and may also be relevant to the control of other filarial worms in the region, such as lymphatic filariasis (Thomson and Connor 2000). The exact effect of [[land cover (Land-use and land-cover change)] changes], especially deforestation, on the composition of the vector population differs from place to place. However, as a net effect an increase of the more virulent vector and therefore an increase of morbidity from onchocerciasis can be noted. |
14.2.1.3.1 Malaria
Deforestation, with subsequent changes in land use (Land-use and land-cover change) and human settlement patterns, has coincided with an upsurge of malaria or its vectors in Africa (Coluzzi et al. 1979, 1984, 1994), Asia (Bunnag et al. 1979), and Latin America (Vittor et al. in press; Tadei et al. 1998). (See Box 14.6.)
|
|
|
Bolívar state in southern Venezuela near the border with Brazil and Guyana covers an area of 24 million hectares (the size of the United Kingdom), 70% of which is forested. It is presently affected by deforestation mainly associated with logging, agriculture, dam construction, and gold mining. Before 1980, malaria was occasionally reported from this state among the indigenous population. Malaria in this area was classified by Gabaldon (1983) as difficult to control since the vector, An. darlingi, was found to bite mostly outside houses (thus harder to spray with insecticides). Also, the human populations were mainly Amerindians with seminomadic habits in remote areas (Gabaldon 1983). During the late 1950s, construction was started on a road to connect Ciudad Bolívar, capital of Bolivar state, to Boa Vista, capital of Roraima state in Brazil. The road construction brought workers from all over the country and opened new opportunities for people seeking land for agriculture, gold and diamond mining, and forest exploitation. In the 1980s, the boom of gold and diamonds attracted a wave of migrants from different parts of the country as well as illegal migrants from Brazil, Guyana, Colombia, and Dominican Republic, among others. Malaria started to increase steadily, reaching the highest peak of over 30,000 cases in 1988, with over 60% cases due to P. falciparum (MSDS 2000). Malaria has since become endemic-epidemic in Bolívar state. Studies carried out in several villages spanning 1999–2000 showed that only three species of anophelines were caught on human landing catches: An. darlingi, An. marajoara, and An. neomaculipalpus (Moreno et al. 2002). In general, biting densities were low (fewer that two bites per person per hour). Nevertheless, there was a strong correlation between An. darlingi density and malaria incidence (P<0.001). In this area, studies on breeding places showed that up to 13 species of anophelines were present, with An. triannulatus being the most abundant. The most productive breeding sites in terms of anophelines species diversity and density were the abandoned mine dug outs, which vary in size from a few meters in diameter to several kilometers, followed by lagoons created by flooding of streams on artificial and natural depressions of terrain (Moreno et al. 2000). |
The capacity of different Anopheles mosquitoes to transmit malaria varies between species. Anopheline species themselves also occupy a variety of ecological niches. An. darlingi in South America, An. gambiae in Africa, and An. dirus in Southeast Asia are the predominant and highly effective vectors in their respective regions.
When tropical forests are cleared for human activities, they are typically converted into agricultural or grazing lands. This process is usually exacerbated by construction of roads, causing erosion and allowing previously inaccessible areas to become colonized (Kalliola and Paitan 1998) and anopheline mosquitoes to invade. Cleared lands and culverts that collect rainwater are far more suitable breeding sites for malaria-transmitting anopheline mosquitoes than forest (Tyssul Jones 1951; Marques 1987; Charlwood and Alecrim 1989). Forest-dwelling Anopheles species either adapt to newly changed environmental conditions or disappear from the area, which offers other anophelines a new ecological niche (Povoa et al. 2001).
14.2.1.3.2 Forest Arboviruses in the Amazon
A wide variety of arboviruses occurs in the Amazon forest, a consequence of the extreme diversity of both arthropod vectors and wild vertebrates.
Thirty-two arbovirus types have been associated with human disease in the Brazilian Amazon region. Of these, four are important in public health because of their link to epidemics: the Oropouche virus (family Bunyaviridae), dengue and yellow fever viruses (Flaviviridae), and Mayaro virus (Togaviridae). It is noteworthy that Oropouche and dengue viruses are associated with human epidemics in urban areas while Mayaro and yellow fever occur in rural areas. All arboviruses (except dengue) that have been isolated in the Brazilian Amazon are maintained within complex cycles in the forest, where many species of blood-sucking arthropods act as vectors and several wild vertebrates act as reservoir hosts.
There have been historical changes in the Amazonian environment due to natural cyclical processes such as climate variability and as the result of human economic and geopolitical activities. The latter, which includes deforestation, construction of dams and highways, and mining, can disrupt to a greater extent the fragile equilibrium of the forest ecosystem, with impacts in the dynamics of virus transmission (Dégallier et al. 1989; Shope 1997; Vasconcelos et al. 2001).
Dam construction has been associated with the emergence of several different arboviruses, some of them responsible for human disease while others were previously unknown (Vasconcelos et al. 2001b).
Comparative studies carried in the 1970s and 1980s in Altamira and Tucurui municipalities prior, during, and after the construction of the Tucurui Dam in the State of Pará, Brazil, showed that inadequate management of the environment can cause an increase in the occurrence of a known virus or the appearance of a new one (Pinheiro et al. 1977; Dégallier et al. 1989). Examples of arboviruses that emerged or reemerged in the Brazilian Amazon region and the factors responsible are shown in Table 14.3.
| Table 14.3. Probable Factors in Emergence of Arbovirus in Brazilian Amazon Region and Association with Human Disease (Vasconcelos et al. 2001) | ||
| Virus | Probable Factors for Emergence | Disease in Humans |
| Dengue | poor mosquito control; increased urbanization in tropics | yes, epidemic |
| Guaroa | flooding of dama | yes, sporadic cases |
| Gamboa | flooding of dama; migrating birds | not yet |
| Mayaro | deforestation | yes, limited outbreak |
| Oropouche | deforestation; increase of colonization and urbanization in Amazon | yes, epidemic |
| Triniti | flooding of dama | not yet |
| Yellow fever | urbanization in tropics; deforestation; lack of widespread immunization | yes, epidemic |
| Anopheles A virusesb | flooding of dama | not yet |
| Changuinola virusesc | flooding of dama; deforestation; use of subsoil | not yet |
| a During construction of dam in Tucurui, Pará State, millions of hematophagous insects were obtained in a few days, from which several strains of previously known and new arboviruses were obtained b In this serogroup of family Bunyaviridae, the new viruses Arumateua, Caraipé, and Tucurui were isolated, and there was also an increase in circulation of Lukuni and Trombetas viruses. c In this serogroup of family Reoviridae, 27 new arboviruses were isolated in Tucurui, 4 in Carajas (use of subsoil), and 8 in Altamira (deforestation for several purposes) from phlebotominae sandflies | ||
Uncontrolled urbanization or colonization near forest areas has been typically associated with the emergence of Oropouche fever and Mayaro fever viruses (Vasconcelos et al. 2001a). The Oropouche virus has been responsible for at least 500,000 infections in the last 40 years in the Amazon, and it is spreading.
Current modifications to the forest ecosystem are likely to result in the spread of infections if vectors and reservoirs find better ecological conditions. This mechanism may explain the large epidemics of Oropouche fever virus in the Brazilian Amazon from 1960 to 1990, as well as in other Latin American countries, especially Peru and Panama (Pinheiro et al. 1998; Watts et al. 1998; Saeed et al. 2000). Alternatively, if the ecological changes are detrimental to the nonhuman carriers of the virus, they will probably disappear due to the absence of the basic elements necessary for their survival. This could explain the absence of many previously identified virus species that are no longer found despite continued surveillance (Vasconcelos et al. 2001a).
Deforestation for agricultural expansion has been the most important factor associated with the spread of yellow fever in Africa and its reemergence in Brazil (in the state of Goiás), although climatic extremes are also important, for instance Brazil in 2000 (Vasconcelos et al. 2001b). Yellow fever is maintained by a sylvatic cycle between primates and mosquitoes in the forest. Evidence from epidemics in Côte d’Ivoire (1982), Burkina Faso (1983), Nigeria (1986 and 1987), and Mali (1987) and other, long-term, studies have established a clear link between deforestation and yellow fever’s increasing area of endemicity (Cordellier 1991). However, it should be noted that yellow fever epidemics occur in urban and dry savanna areas as well and can be transmitted by different mosquito species more adapted to these environments.
14.2.1.4 Habitat Change and Infectious Diseases in Urban Systems
Uncontrolled urbanization has many adverse human health consequences, primarily due to health infrastructure problems and overcrowded, unsanitary conditions. This section focuses on infectious diseases linked to urbanization.
14.2.1.4.1 Lymphatic filariasis
Lymphatic filariasis is one of the most prevalent tropical diseases, with some 120 million people infected, primarily in India and Africa, where there has been no decline in the incidence of the disease for the past decade. The disease is reported to be responsible for 5 million DALYs lost annually, ranking third after malaria and tuberculosis (WHO/TDR 2002).
Distribution and transmission of the lymphatic filariasis are closely associated with socioeconomic and behavioral factors in endemic populations. In Southeast Asia, urban Bancroftian filariasis Wuchereria bancrofti infection, a filarial nematode, is related to poor urban sanitation, which leads to intense breeding of C. quiquefasciatus, its principal mosquito vector (Mak 1987). In Sri Lanka, C. quinquefasciatus was not originally present in a forested environment, but rapidly invaded as soon as forest clearing began and settlement expanded (Asmerasinghe in press). In urban East Africa, filariasis is also transmitted by C. quinquefasciatus, but in rural areas across tropical Africa it is spread by the same anopheline mosquito species that transmit malaria (Manga 2002).
14.2.1.4.2 Dengue fever
In terms of morbidity and mortality, dengue fever—caused by a virus that has four serotypes or genetic variants—is the most important human viral disease carried by mosquitoes. It is caused by a flavivirus and is endemic in about 100 countries and found in all continents except Europe (WHO/TDR 2004). Around 80 million cases are reported every year, of which 550,000 people need hospital treatment and about 20,000 die. Although dengue is primarily a tropical disease, it has become a great concern in countries with temperate climates because of an increased number of imported cases, resulting from increased air travel and the introduction of Aedes albopictus, an exotic vector adapted to a cold climate (Kuno 1994).
However, the primary vector of large dengue epidemics is Ae. aegypti, a day-biting mosquito that prefers to feed on humans and that breeds at sites typically found in the urban environment: discarded tires, cans, and other trash that accumulates rainwater; water storage devices in houses; flower pots and even plants that collect water. This species has made extraordinary evolutionary adjustments to coexist with human beings since its origins in Africa as a forest species feeding principally on wild animals (rodents and so on) and laying eggs in tree holes containing rainwater. One subspecies—Ae. aegypti aegypti—evolved to become highly adapted to indoor or peridomestic environs, breeding in artificial containers, and followed humankind on its journeys and migrations throughout the globe (Monath 1994).
The spread of the disease is associated with the geographical expansion of the vector species, favored by current housing and water supply conditions as well as garbage collection practices in developing countries. Therefore, uncontrolled urbanization, which is frequently associated with population growth and poverty (resulting in substandard housing and inadequate water and waste management systems), plays a major role in creating the conditions for dengue epidemics. Analysis of associations between social and economic variables, such as income and education level, in residential urban areas and the incidence of dengue infection has shown that low-income neighborhoods have the highest levels of infection (Costa and Natal 1998). Moreover, communities with high infestations of Ae. aegypti, especially if they are near forests that maintain yellow fever virus, face a significant risk of yellow fever epidemics.
14.2.1.4.3 Other diseases linked to urbanization
Soil disturbance from building construction in arid environments can encourage coccidioidomycosis, a fungal pneumonia. Increased aridity and eventual desertification due to increasing global [[temperature]s] may increase the potential for infection. Coccidioidomycosis is spread by dust, and disease outbreaks are often preceded by increased rain, followed by dry periods, and especially in the wake of soil disturbances. A well-documented outbreak followed the 1994 Northridge, California, earthquake, when 317 cases resulted (Schneider et al. 1997). In a study in Greece, factors thought to contribute to the extraordinary increases in cases were a drought lasting five to six years, abundant rain in 1991 and 1992, construction of new buildings, and arrival of new susceptible residents to the endemic areas (Pappagianis 1994)
Leishmaniasis has been associated with urban settlements in forested regions and is widespread in 22 countries in the New World and in 66 in the Old World (WHO, TDR 2004). There are two major types of leishmaniasis: cutaneous and visceral (kala-azar). Cutaneous leishmaniasis is originally a forest disease but may adapt to urban settings with some vegetation and cycling in dogs.
Uncontrolled urbanization or colonization near forest areas has been typically associated with the emergence of Oropouche fever and Mayaro fever viruses (Vasconcelos et al. 2001a). The relationship between malaria and urbanization in two cities is presented in Box 14.7.
|
BOX 14.7 Malaria and Urbanization in Brazil |
|
The transmission of malaria in urban areas in the Americas occurs when urbanization invades the habitat of vectors. These phenomena have been observed in cities of Brazil such as Belém (Pará state) located near the mouth of the Amazon River and in Manaus (Amazonas state). In a recent study comparing malaria transmission and epidemiology over 60 years in Belém, Póvoa et al. (2002) showed that the incriminated vectors in the 1940s, An. aquasalis and An. darlingi, are still currently important vectors. The anopheline species diversity has increased from 2 in the 1930s to 6 in the 1940s to 10 in the 1990s. An. darlingi was eliminated from Belém in the 1960s and was absent for approximately 20 years, probably due to the destruction of breeding sites in forested areas as urbanization increased. During this period the reported malaria cases were attributed to human immigration from rural areas into Belém (Marques 1986) or people from Belém traveling to endemic areas during the holidays and returning with malaria parasites in their blood (Souza 1995). Nevertheless, An. aquasalis was present in coastal areas influenced by tides. The population of Belém increased from 206,331 in 1942–43 to 934,322 in 1980–89 and then to 1,367,677 in 1996. The number of malaria cases went from 363 in 1942–43 to 1,197 in 1980–89 and 2,716 in 1996. Approximately 80% of the original forest has been destroyed (Povoa et al. 2002), but the expansion of the city (particularly the District of Daent) toward the forested protected area and low coastal swampy areas provided new larval habitats, resulting in a higher mosquito diversity and closer proximity between human dwellings and mosquito habitat, which increases the transmission of malaria. A similar situation has occurred in Manaus, where the process of urbanization eliminated An. darlingi from the city by 1976 (Tadei et al. 1998). Nevertheless, the accelerated expansion of the suburbs into the surrounding jungle reestablished the contact between the human population and the principal vector, An. darlingi, which by 1988 colonized the city again and triggered malaria epidemics. Vectors are also adapting to new circumstances; for example, mosquitoes have varied their feeding time in areas where humans have intervened (Tadei et al. 1998). At present, malaria cases continue to be reported from the periurban areas of Manaus. |
14.2.1.5 Ecological Change and Infectious Disease in Coastal and Freshwater Systems
Cholera and severe forms of gastroenteritis are caused by Vibrio cholerae and V. parahaemolyticus. In tropical areas, cases are reported year-round. In temperate areas, cases are mainly reported in the warmest season. The seventh cholera pandemic is currently spreading across Asia, Africa, and South America. In 1992, a new strain or serogroup (V. cholerae O139) appeared and has been responsible for epidemics in Asia. During the 1997/98 El Niño, excessive flooding caused cholera epidemics in Djibouti, Somalia, Kenya, Tanzania, and Mozambique. Warming of the African Great Lakes due to climate change may create conditions that increase the risk of cholera transmission in the surrounding countries.
Pathogens are often found in coastal waters, and transmission occurs through consumption of shellfish or brackish water or through bathing. Coastal waters in both industrial and developing countries are frequently contaminated with untreated sewage (see Chapter 19 (Ecosystems and Human Well-Being: Volume 1: Current State and Trends: Human Infectious Disease Agents) 2), and warmth encourages microorganism proliferation. The presence and increased transmission of Vibrio spp. (some of which are pathogens that cause diarrhea), such as V. vulnificus, a naturally occurring estuarine bacterium, has been associated with higher sea surface temperatures. Phytoplankton organisms respond rapidly to changes in environmental conditions and are therefore sensitive biological indicators of the combined influences of climate change and soil and water pollution. Algal blooms are associated with several environmental factors, including sunlight, pH, ocean currents, winds, sea surface [[temperature]s], and runoff (which affects nutrient levels), as described in Chapters 12 (Ecosystems and Human Well-Being: Volume 1: Current State and Trends: Human Infectious Disease Agents) and 19 (Ecosystems and Human Well-Being: Volume 1: Current State and Trends: Human Infectious Disease Agents).
V. cholerae and other gram-negative bacteria can be harbored in many forms of algae or phytoplankton. V. cholerae can assume a noncultivable but viable state, returning to a cultivable, infectious state with the same conditions that promote algal blooms (Colwell 1996). Some species of copepod zooplankton apparently provide an additional marine reservoir for V. cholerae, facilitating its long-term persistence in certain regions, such as in the estuaries of the Ganges and Brahmaputra in India. According to this theory, the seasonality of cholera epidemics may be linked to the seasonality of plankton (algal blooms) and the marine food chain. Studies using remote sensing data of chlorophyll-containing phytoplankton have shown a correlation between cholera cases and sea surface temperatures in the Bay of Bengal. Interannual variability in cholera incidence in Bangladesh is also linked to El Niño/Southern Oscillation and regional temperature anomalies (Lobitz et al. 2000), and cholera prevalence has been associated with progressively stronger El Niño events spanning a 70-year period (Rodo et al. 2002).
14.2.2 Niche Invasion or Interspecies Host Transfer Effects on Infectious Disease Transmission
The emergence of many diseases has been linked to the interface between tropical forest communities, with their high levels of biodiversity, and agricultural communities, with their relatively homogenous genetic makeup but high population densities of humans, domestic animals, and crops. For instance, expanding ecotourism and forest encroachment have increased opportunities for interactions between wild nonhuman primates and humans in tropical forest habitats, leading to pathogen exchange through various routes of transmission (Wolfe et al. 2000). (See Box 14.8.)
|
|
|
Zoonotic pathogens are the most significant cause of emerging infectious diseases affecting humans in terms of both their proportion and their impact. Some 1,415 species of infectious organisms are known to be pathogenic to humans; 61% of these are zoonotic and 75% of those considered as emerging pathogens are zoonotic (Taylor et al. 2001). More important, zoonotic pathogens cause a series of EIDs with high case fatality rates and no reliable cure, vaccine, or therapy (such as Ebola virus disease, Nipah virus disease, and hantavirus pulmonary syndrome). They also cause diseases that have the highest incidence rates globally (such as AIDS). AIDS is a special case, because it is caused by a pathogen that jumped host from nonhuman primates and then evolved into a new virus. Thus it is essentially a zoonosis (Hahn et al. 2000) and is thought to have infected the highest number of human individuals of any disease in history. Viruses such as Junin, Machupo, and Guanarito hemorrhagic fever agents in Argentina, Bolivia, and Venezuela, transmitted to humans through the urine of wild rodents, came through the expansion of agricultural practices to new areas; hantaviruses, initially recognized in Korea, came through the urine of infected rodents and were then identified in Asia and Europe. The Junin virus, which causes a hemorrhagic fever, also emerged when the production of vegetables that serve as food source for rodents increased in Argentina in 1957. Because of the key role of zoonoses in current public health threats, wildlife and domestic animals play a key role in the process by providing a ‘‘zoonotic pool’’ from which previously unknown pathogens may emerge (Daszak et al. 2001). The influenza virus is an example, which causes pandemics in humans after periodic exchange of genes between the viruses of wild and domestic birds, pigs, and humans. Fruit bats are involved in a high-profile group of EIDs that include rabies and other lyssaviruses, Hendra virus, Menangle virus (Australia), and Nipah virus (Malaysia and Singapore). This has implications for further zoonotic disease emergence. A number of species are endemic to remote oceanic islands, and these may harbor enzootic and potentially zoonotic pathogens (Daszak et al. 2000). The current major infectious disease threats to human health are therefore emerging and reemerging diseases, with a particular emphasis on zoonotic pathogens jumping host from wildlife and domestic animals. A common, defining theme for all EIDs (of humans, wildlife, domestic animals, and plants) is that they are driven to emerge by anthropogenic changes to the environment. Because threats to wildlife habitat are so extensive and pervading, it follows that many of the currently important human EIDs (such as AIDS and Nipah virus disease) are driven by anthropogenic changes to wildlife habitat such as encroachment, deforestation, and others. This is essentially a process of natural selection in which anthropogenic environmental changes perturb the host-parasite dynamic equilibrium, driving the expansion of those strains suited to the new environmental conditions and driving expansion of others into new host species (Cunningham et al. in press). The selection process acts on the immense pool of varied pathogen strains circulating within the population (c.f. the ‘‘zoonotic pool,’’ Morse 1993). Thus, very few EIDs are caused by newly evolved pathogens, although notable exceptions include drug-resistant pathogens, newly re-assorted influenza strains, and pathogens with point mutations that increase their virulence (such as canine parvovirus, Parrish et al. 1985). Even in these examples, it is possible that the new strains were already present in the pathogen population. For example, recent work shows that drug-resistant strains of some common microbes circulate within rodent populations in areas outside normal contact with antibiotics (Gilliver et al. 1999). |
14.2.2.1 Cultivated Systems and Niche Invasion
Intensive farming practices have seen the emergence of several devastating herd and flock diseases, including Nipah virus, bovine spongiform encephalopathy, foot and mouth disease, severe acute respiratory syndrome, and avian influenza.
14.2.2.1.1 Nipah virus in Malaysia
The emergence of many diseases can be viewed as a pathogen invading a new or recently vacated niche. For example, Nipah virus emerged in Malaysia in 1999, causing over 100 human deaths (Chua et al. 2000). This highly pathogenic virus (with a case fatality rate greater than 40%) was previously unknown as a human pathogen, and emerged from its natural reservoir hosts (fruit bats) via domestic animal (pig) amplifier hosts.
The ecological changes that caused this virus to invade a new niche appear to be a complex series of anthropogenic alterations to fruit bat habitat and agriculture set in a background of increasing ENSO-caused drought (Daszak et al. 2001; Field et al. 2001; Chua et al. 2002). First, the virus appears not to be able to move directly from bats to humans, so the development of a pig industry in Malaysia has been a crucial driver of emergence. Second, fruit bat habitat has been largely replaced in peninsular Malaysia by crop plants such as oil palm. Third, deforestation in Sumatra coupled with increasing amplitude, intensity, and duration of ENSO-driven drought has led to repeated, significant seasonal haze events that cover Malaysia. These events reduce the flowering and fruiting of forest trees that are the natural food of fruit bats and may have changed the fruit bats’ migration patterns and ability to find food.
These interrelated events are theorized to have coincided just prior to the Nipah virus outbreaks, when the large mid-1990s ENSO event was associated with a drop in fruit production and the appearance of Pteropus vampyrus (the key Nipah virus reservoir) for the first time at the index farms, where Nipah virus was initially identified (Chua et al. 2002).
14.2.2.1.2 Bovine spongiform encephalopathy (‘‘mad cow’’ disease)
The background of the BSE epidemic is well known. In order to improve the protein content in the diet of cattle, ground-up sheep and cattle remains—including of the brain and spinal cord—were fed to cattle. This practice was claimed as economically rational because it turned a waste product into a valuable food. But from an ecological perspective it was anything but rational; cattle are normally vegetarian, and are certainly not cannibals (Prusiner 1997).
While it was originally argued that this practice is harmless, a similar disease in sheep (scrapie) was known for centuries, and one in humans (kuru), transmitted through the ritual cannibalism of human brains, was already known in New Guinea. In time, the causal agent of BSE, an unusual protein called a prion, was transmitted to humans, causing a devastating, rapidly progressive, still untreatable brain disease, called new variant Creutzfeldt- Jakob disease. So far, the size of this human epidemic has been modest, but transmission is probably still occurring through blood transfusions (Llewelyn et al. 2004) and surgical instruments that cannot be sterilized. As well as these human health effects, the BSE epidemic had an immense economic and psychological cost to farmers, as millions of cattle were slaughtered prematurely.
14.2.2.1.3 Severe acute respiratory syndrome
SARS gained international attention during an outbreak that began sometime during November 2001 in China. Wet markets, a known source of influenza viruses since the 1970s, were found to be the source of the bulk of the infections (Webster 2004). (Live-animal markets, termed ‘‘wet markets,’’ are common in most Asian societies and specialize in many varieties of live small mammals, poultry, fish, and reptiles (Brieman et al. 2003).) The majority of the earliest reported cases of SARS were of people who worked with the sale and handling of wild animals. The species at the center of the SARS epidemic are palm civet cats (Paguna larvata), raccoon dogs (Nyctereutes procuyoinboides), and Chinese ferret badgers (Melogale moschata) (Bell at al. 2004). As of July 2003, there had been 8,096 cases and 774 deaths reported worldwide (WHO 2004).
14.2.2.1.4 Avian influenza
Avian influenza virus has caused fatalities in humans, highlighting the potential risk that this type of infection poses to public health (Capua and Alexander 2004). Genetic reassortment within a person coinfected with human and avian strains of influenza virus could potentially link the high transmissibility associated with human-adapted viruses with the high rates of mortality observed in the avian cases, thus triggering a potentially devastating pandemic (Ferguson et al. 2004). The ‘‘Spanish flu’’ pandemic of 1918–19 was the largest infectious disease event in recorded history, killing over 20 million people. Recently a single gene coding for the viral haemagglutinin protein from the 1918 pandemic influenza A strain was identified and produced extraordinary virulence in a mouse model (Kobasa et al. 2004). Such highly virulent recombinant viruses will continue to pose a threat through agricultural practices.
14.2.2.2 Forest Systems and Interspecies Host Transfer
14.2.2.2.1 Bushmeat hunting and disease emergence
The global trade in bushmeat is quite extensive. In Central Africa, 1–3.4 million tons of bushmeat are harvested annually (Fa and Peres 2001). Also, in West Africa, a large share of protein in the diet comes from bushmeat; in Côte d’Ivoire, for example, 83,000 tons are eaten each year (Feer 1993) and in Liberia, 75% of meat comes from wildlife (105,000 tons consumed a year) (Fa and Peres 2001). The bushmeat harvest in West Africa includes significant numbers of primates, so the opportunity for interspecies disease transfer between humans and non-human primates is not a trivial risk.
Contact between humans and other animals can provide the opportunity for cross-species transmission and the emergence of novel microbes into the human population. Road building is linked to the expansion of bushmeat consumption that may have played a key role in the early emergence of human immunodeficiency virus types 1 and 2 (Wolfe et al. 2000). Simian foamy virus has been found in bushmeat hunters (Wolfe et al. 2004), and workers collecting and preparing chimpanzee meat have become infected with Ebola (WHO 1996). The initiation of a local epidemic of monkeypox (an orthopoxvirus similar to smallpox), which continued for four generations of human-to-human contact, has been attributed to the hunting of a red colobus monkey (Jezek et al. 1986). Also, there are other specific human activities that pose risks similar to those of wildlife hunting and butchering. For example, in the Tai forest in Côte d’Ivoire, a researcher performing a necropsy on a chimpanzee contracted Ebola (Le Guenno et al. 1995).
The Taxonomic Transmission Rule states that the probability of successful cross-species infection increases the closer hosts are genetically related (chimpanzees are closer genetically to humans, for example, than birds or fish are), since related hosts are more likely to share susceptibility to the same range of potential pathogens (Wolfe et al. 2000). Surveillance of nonhuman primates— reservoirs or sources for microbial emergence—can flag emerging pathogens (Wolfe et al. 1998).
14.2.2.2.2 Diseases transferred from human hosts to wildlife
Cross-species transmission also increases the probability that endangered nonhuman primate species and other wildlife will come into contact with human pathogens. The parasitic disease Giardia was introduced to the Ugandan mountain gorilla, Gorilla gorilla beringei, by humans through ecotourism and conservation activities (Nizeyi et al. 1999). Gorillas in Uganda also have been found with human strains of Cryptosporidium parasites, presumably from ecotourists (Graczyk at al 2001). The invasion of human tuberculosis into the banded mongoose (Alexander et al. 2001) represents another case of a human pathogen invading a free-ranging wildlife species.
These host transfer and emergence events not only affect ecosystem function, they could possibly result in a more virulent form of a human pathogen circling back into the human population from a wildlife host and may also allow the development of other transmission cycles to develop outside human-to-human contact.
14.2.3 Biodiversity Change Effects of Infectious Disease Transmission
Biodiversity change includes issues of species replacement, loss of key predator species, and variation in species population density. (See Chapter 4 (Ecosystems and Human Well-Being: Volume 1: Current State and Trends: Human Infectious Disease Agents).) The equilibrium among predators and prey, hosts, vectors, and parasites is an essential service provided by ecosystems. (See Chapter 11 (Ecosystems and Human Well-Being: Volume 1: Current State and Trends: Human Infectious Disease Agents).) It plays the role of controlling the emergence and spread of infectious diseases, although it is only recently that this protective function of biodiversity has been acknowledged (Chivian 2001).
14.2.3.1 Biodiversity Change at the Forest/Urban System Interface
Lyme disease can be used as a model system to illustrate some effects of biodiversity change on infectious disease transmission. In eastern U.S. oak forests, studies on the interactions between acorns, white-footed mice (Peromyscus leucopus), moths, deer, and ticks have linked defoliation by gypsy moths with the risk of Lyme disease (Jones et al. 1998). Most vectors feed on a variety of host species that differ dramatically in their function as a reservoir— that is, their probability of transmitting the infection from host to vector. Increasing species richness has been found to reduce disease risk (Schmidt and Ostfeld 2001), and the involvement of a diverse collection of vertebrates in this cases may dilute the impact of the main reservoir, the white-footed mouse (Ostfeld and Keesing 2000a).
Habitat fragmentation also plays a part in disease emergence; mouse populations that are isolated in fragments seem to fluctuate, unregulated by biotic interactions. Moreover, predators tend to be absent in small woodlots, and probable competitors occur at lower densities in these areas than in more continuous habitat. Therefore, habitat fragmentation causes a reduction in biodiversity within the host communities, increasing disease risk though the increase in both the absolute and relative density of the primary reservoir.
The same conclusions may apply to a number of other diseases, including cutaneous leishmaniasis, Chagas disease, human granulocytic ehrlichiosis, babesiosis, plague, louping ill, tularemia, relapsing fever, Crimean Congo hemorrhagic fever, and LaCrosse virus (Ostfeld and Keesing 2000b).
14.2.3.2 Biodiversity Change in Forest Systems: Variation in Population Density
Most of the human cases of rabies acquired from bat bites in Brazil result from isolated attacks, usually by sick bats that drop from their resting places or fly into houses and are unwisely grasped by a person. But over the last few decades several reports have been published of outbreaks of vampire bat (Desmodus rotundus) attacks on humans in Latin America, occasionally transmitting rabies (Thomas and Haran 1981; Schneider and Burgoa 1995).
Recently compiled data show that vampire bats were the second most frequent species transmitting rabies to humans in Brazil (25% of all cases) in 1993, second only to dogs (Schneider and Burgoa 1995); this was also true previously in Mexico (Anonymous 1991). In the majority of vampire bat attacks, the underlying motive for the attacks seemed to be the same: bat populations deprived of their abundant and readily obtained primary food sources (animals) sought alternative hosts (humans) to feed on. In rural areas, it has happened when animals such as cattle or pigs were rapidly eliminated from an area (MacCarthy 1989; Schneider 1991; Lopez 1992; Costa et al. 1993).
Similar attacks can occur when wild vertebrates are reduced following establishment of human settlements in remote areas (Almansa and Garcia 1980; Schneider 1991). For example, massive attacks by bats have occurred in the gold mining camps in the Amazon, where wild animal species that served as food sources for the bats were depleted due to overhunting or were chased away by the noise produced by water pumps and airplanes (Uieda et al. 1992; Coelho 1995; Schneider et al. 1996; Confalonieri 2001).
In this context, bat attacks on humans are a direct consequence of human-induced environmental modifications caused by the elimination of native species of animals in natural landscapes, with a consequent host shift and an increase in the likelihood of rabies transmission.
14.2.4 Human-induced Genetic Changes of Disease Vectors or Pathogens
One of the key properties of microbes that have successfully managed a ‘‘host transfer,’’ migrating into a new ecological niche, is the potential for mutability. This mutagenic potential of the microbe is exploited once selection pressure (through ecological change) is exerted on the microbe. For example, Brault et al. (2004) have associated genetic shifts in the Venezuelan encephalitis virus with epidemics in animal hosts in southern Mexico due to extensive deforestation and habitat changes, where the subsequent replacement of mosquito vector species is exerting new evolutionary pressures on the virus.
14.2.4.1 Resistant Bacteria from the Use of Antibiotics in Animal Feed
Intensive animal production (both agriculture and aquaculture) has many impacts on ecosystems and human well-being. (See Chapters 19 (Ecosystems and Human Well-Being: Volume 1: Current State and Trends: Human Infectious Disease Agents) and 26 (Ecosystems and Human Well-Being: Volume 1: Current State and Trends: Human Infectious Disease Agents).) Antibiotics are routinely used for prophylaxis and growth promotion in high-production livestock agriculture rather than being used sparingly for medical purposes. Such subtherapeutic levels exert selective pressure on the emergence of resistant bacteria. Campylobacter bacteria sampled from pigs in South Australia, for example, show widespread resistance (60– 100%) to antibiotics such as erythromycin, ampicillin, and tetracycline, and E. coli strains showed widespread resistance to multiple antibiotics (Hart et al. 2004). Livestock have also been shown to be reservoirs of drug-resistant Salmonella bacteria (Busani et al. 2004) and other E. coli that are resistant even to newergeneration antibiotics, like cephalosporins (Shiraki et al. 2004).
Salmonella enteritidis likely stemmed from antibiotic prophylaxis in the poultry industry that led to removal of S. gallinarum and S. pullorum during the 1960s and the creation of a vacant niche in the gut, which was subsequently filled by S. enteritidis. These three pathogens share a common surface antigen; thus, flock immunity prior to removal of the former two species would have prevented the latter from becoming established.
14.2.4.2 Genetic Changes in Disease Vectors at Forest/Urban Interface
Chagas disease is a deadly disease transmitted by triatomine beetles in South and Central America. The niche and trophic relationships of these insects have direct epidemiological importance, and pesticide resistance is changing the ecology and transmission of this disease.
Only a few cases of typical insecticide resistance have been reported (Busvine 1970; Cockburn 1972), but important niche adaptations to houses and domestic areas seem to have taken place, particularly for the Triatoma infestans beetle (Dujardin et al. 1997a, 1997b; Gorla et al. 1997; Noireau et al. 1997; Panzera et al. 1997; Schofield et al. 1997). Domiciliary adaptation generally involves genetic simplification (Schofield 1994; Dujardin et al. 1998; Rabinovich et al. 2001), including the loss of genetic material that may make triatomines highly susceptible to chemical control (Borges et al. 1999) and may cause simplification of some specific characteristics, such as a reduction in body size and sexual dimorphism (Steindel 1999). Specific markers can differentiate populations that have survived chemical control from invasive populations (Costa 1999).
Triatomine ‘‘ecological successions’’ (replacement of some species by other species) follows control programs and environmental changes such as deforestation (Costa 1999). For instance, T. sordida has progressively replaced T. infestans because of elimination of the latter from houses. And T. pseudomaculata, which is predominantly peridomiciliary (around dwellings), has replaced T. brasiliensis in some areas treated with insecticides (Diotaiuti et al. 1998). The Brazilian Amazon, where Chagas disease was always considered endemic in wild animals, presently has at least 18 triatomine species reported, 10 of which are infected by Trypanosoma cruzi, the deadly human form of the disease (Coura et al. 2002; Teixeira et al. 2000).
14.2.5 Environmental Contamination of Infectious Agents of Diseases
14.2.5.1 Cryptosporidiosis
Cryptosporidium, a protozoan that completes its life cycle within the intestine of mammals, sheds high numbers of infectious oocysts that are dispersed in feces. One hundred and fifty-two species of mammals have been reported to be infected with C. parvum, which causes diarrhea and gastrointestinal illness. C. parvum infections of humans were first reported in 1976 and have since been reported from 90 countries. It is highly prevalent in ruminants and readily transmitted to humans. Cryptosporidium oocysts are very small (~ 3 microns) and are difficult to remove from water; another study found 13% of treated water in the United States still contained Cryptosporidium oocysts, indicating some passage of microorganisms from source to treated drinking water (LeChevallier and Norton 1995).
In a survey of farms in the state of Pennsylvania, 64% returned at least one bovine stool sample that was positive for C. parvum, and all samples were positive at 44% of farms. All cattle had full access to water courses that could be contaminated with these parasites (Graczyk et al. 2000). Environmental factors such as land use, climate extremes, and inadequate water treatment are now recognized as contributing factors in the spread of cryptosporidiosis (Rose et al. 2002).
The extent of the problem of C. parvum in tropical environments where water supplies are less well controlled is unknown. However, the impact of changing climate patterns and increased frequency of severe weather events on watershed and water storage facilities is likely to increase the level of oocyst contamination (Graczyk et al. 2000).
14.2.5.2 Leptospirosis
Leptospirosis is a bacterial waterborne disease disseminated by mammals, which shed the pathogen in their urine. Leptospirosis occurs across the globe. In tropical areas, the disease usually occurs as outbreaks during the rainy season, due to the flooding of densely populated low-lying areas infested with domestic rats, which breed prolifically because of poor garbage collection practices. In Rio de Janeiro, for example, epidemics have always been associated with seasonal increases in rainfall (Roberts et al. 2001). Outbreaks in rural areas are not as common as in urban areas.
14.2.6 Synergies between Malnutrition and Infectious Diseases
Malnutrition, as a consequence of environmental degradation, has a huge global impact on morbidity and mortality due to infectious diseases. For children under five years of age in the developing world, being underweight equates to about half the mortality risk of the main infectious diseases such as diarrhea, malaria, pneumonia, and measles. (See [[Chapter 8 (Ecosystems and Human Well-Being: Volume 1: Current State and Trends: Human Infectious Disease Agents)]2] for more on the health impacts of malnutrition). (See also Box 14.9.)
|
|
|
Nomads often face a different spectrum of health problems than nonnomadic populations. Their adaptation to a specific environment—often one that supports human settlement only on a transient basis—makes them sensitive indicators of [[land cover (Land-use and land-cover change)] changes]. Important inequalities between nomadic and nonnomadic populations exist not only with regard to the disease spectrum, but even more important with regard to access to health care (Sheik-Mohamed and Velena 1999). Nomadic lifestyle interferes with issues of compliance and adherence, but health care as an instrument of control used by settled populations is also an issue. Primary health care for nomads would have to be adapted to their lifestyle and needs. Continuous drought, for example, may force nomads into living conditions that threaten their health as well as their whole lifestyle (Loutan and Lamotte 1984). Becoming sedentary carries a major risk of mortality and infectious disease for nomadic populations in the African rain forest. Nomadic populations live in the semiarid regions of the African continent or in the rain forest regions of Africa, South America, and Asia. Two big groups that can be distinguished are pastoralists (in the semiarid regions) and hunters/collectors (in rain forests). Kalahari Bushmen or Australian Aborigines would also be part of the hunters/collectors group. For nomadic populations in the semiarid regions of Africa, water supply and management is a crucial issue. Water is also an essential part in many land cover changes and is of course essential to health. Child mortality in the Sahel region of Africa was found to be higher, and general access to health care was found to be limited. Infectious disease prevalence in pastoralists differs from settled populations with a profile that is directed toward diseases that have a reservoir in cattle, such as tuberculosis or brucellosis, and those that require long-term treatment, such as some sexually transmitted diseases and again tuberculosis (Niamir-Fuller and Turner 1999). Pygmies in the Central African rain forest have been protected against some infectious diseases (including HIV1) due to their partial isolation. However, they are fully susceptible when coming into contact with carriers. Major land cover changes like deforestation in the Central African basin increase contact between different populations. Infections that require long-term treatment, like leprosy or tuberculosis, are difficult to target in nomadic populations in the rain forest. |
14.3 New Tools and Methods for Assessment of Ecosystem Change and Human Disease
Table 14.4 summarizes the published literature on the link between ecological change and human disease. New tools applied across these ecologically linked diseases will improve understanding of these linkages and of the emergence of infectious diseases. Time-series analysis, geographic information systems, and spatial analysis (which encompass a range of technologies and approaches, including digital mapping, analysis of remotely sensed imagery, spatial statistics, ecological niche modeling, and the use of global positioning systems), for example, have proved useful in studying diseases emerging from land use (Land-use and land-cover change) change. (See also Chapter 3 (Ecosystems and Human Well-Being: Volume 1: Current State and Trends: Human Infectious Disease Agents).) There have also been a number of significant reviews of tools that have been applied specifically to public health (Beck et al. 2000; Thomson and Connor 2000; Hay 2000).
| Table 14.4. Infectious Diseases and Mechanisms of Potential Changing Incidence as Related to Ecosystem Changes | |||||||
| Disease | Cases per Year |
DALYsa | Emergence Mechanism |
Anthropogenic Drivers |
Geographical Distribution |
Expected Variation from Ecological Change |
Confidence Level |
| (thousand) | |||||||
| Malaria | 350 million | 46,486 | niche invasion; vector expansion |
deforestation; water projects |
tropical (America, Asia, and Africa) |
++++ | +++ |
| Lymphatic filariasis | 120 million | 5,777 | habitat alteration | water projects; urbanization |
tropical America and Africa |
+ | +++ |
| Schistosomiasis | 120 million | 1,702 | intermediate host expansion |
dam building; irrigation | America; Africa; Asia |
++++ | ++++ |
| Dengue fever | 80 million | 616 | vector expansion | urbanization; poor housing conditions |
tropical | +++ | ++ |
| HIV | 42 million | 84,458 | host transfer | forest encroachment; bushmeat hunting; human behavior |
global | + | ++ |
| Onchocerciasis | 18 million | 484 | habitat alteration | spillways of dams | Africa; tropical America |
++ | +++ |
| Chagas disease | 16-18 million | 667 | habitat alteration | deforestation; urban sprawl and encroachment |
Americas | ++ | +++ |
| Leishmaniasis | 12 million | 2,090 | host transfer; habitat alteration |
deforestation; agricultural development |
tropical Americas; Europe and Middle East |
++++ | +++ |
| Meningitis | 223,000 (in 2002) |
6,192 | habitat alteration; dust storms |
desertification | Saharan Africa | ++ | ++ |
| Rabies | 35,000 deaths |
1,160 | biodiversity loss, altered host selection |
deforestation and mining | tropical | ++ | ++ |
| Trypanosomiasis | 30,000– 500,000 |
1,525 | habitat alteration | deforestation | Africa | +++ | ++ |
| Japanese encephalitis |
30,000– 50,000 |
709 | vector expansion | irrigated rice fields | Southeast Asia | +++ | +++ |
| Rift Valley fever | 27,500 (Kenya 1998) |
heavy rains | climate variability and change; dam building |
Africa; Middle East |
+++ | ++ | |
| Lyme disease | 23,763 (U.S. 2002) |
depletion of predators; biodiversity loss; reservoir expansion |
habitat fragmentation | North America and Europe |
++ | ++ | |
| SARS | 8,098e | host transfer | intensive livestock | global | + | + | |
| West Nile virus and other encephalitides |
5,483 (U.S. average 2002–04) |
– | niche invasion | international travel; climate variability |
Americas; Eurasia |
++ | + |
| BSE | 133d | host transfer | intensive livestock farming |
Europe | + | + | |
| Cholera | b | c | sea surface temperature rising |
climate variability and change |
global (tropical) | +++ | ++ |
| Cryptosporidiosis | b | c | contamination by oocystes |
poor watershed management where livestock exist |
global | +++ | ++++ |
| Coccidioidomycosis | – | – | disturbing soils | climate variability | global | ++ | +++ |
| Ebola | – | forest encroachment; bushmeat hunting |
forest encroachment | Africa | + | + | |
| Guanarito; Junin; Machupo |
– | – | biodiversity loss; reservoir expansion |
monoculture in agriculture after deforestation |
South America | ++ | +++ |
| Oropouche/ Mayaro virus in Brazil |
– | – | vector expansion | forest encroachment; urbanization |
South America | +++ | +++ |
| Leptospirosis | – | – | habitat alteration | agricultural development; urban sprawl |
global (tropical) | ++ | +++ |
| Nipah/Hendra viruses |
– | niche invasion | industrial food production; deforestation; climate abnormalities |
Australia; Southeast Asia |
+++ | + | |
| Salmonellosis | – | niche invasion | antibiotic resistance from using antibiotics in animal feed |
global | + | + | |
| Key: + low + + moderate + + + high + + + + very high | |||||||
| a Disability-adjusted life year: years of healthy life lost, a measure of disease burden for the gap between actual health of a population compared with an ideal situation where everyone lives in full health into old age. (WHO World Health Report 2004) b and c Diarheal disease (aggregated) deaths and DALYs respectively: 1,798 ? 1,000 cases and 61,966 ? 1,000 DALYs. d Human cases from 1995 to 2002. e From November 2002 to July 2003, probable cases of SARS reported to the World Health Organization. | |||||||
Ecological niche modeling is an approach used in biogeography to predict the distributional range of species from existing occurrence data (Anderson et al. 2003). Using genetic algorithms as a decision tool in a GIS containing layers of environmental information (such as topography, climate, and vegetation), epidemiological and spatial risk stratification can be achieved from data on the location of vectors or pathogens. This approach has been successfully used in the case of Chagas disease and for vectors of leishmaniasis and filovirus infections (Peterson et al. 2002, 2004a, 2004b).
Increasingly in recent years, meteorological satellite data have been used to help model the spatial and seasonal dynamics of disease transmission and develop early warning systems (Connor et al. 1998). These relatively low cost and easy-to-use data sources have become familiar to public health services in Africa. For example, environmental data indicating areas at risk of malaria epidemics are beginning to be routinely incorporated into the WHO/UNICEF-supported disease surveillance software Health-Mapper that is widely used by ministries of health (WHO 2001).
References
- Akogbeto, M., 2000: Lagoonal and coastal malaria at Cotonou: entomological findings. Sante, Jul-Aug., 10(4), 267–275.
- Alexander, K.A., E. Pleydell, M. Williams, J.F.C. Nyange, A.L. Michel, 2001: The zoonotic importance of mycobacterium tuberculosis: a potential threat to free-ranging wildlife populations? Journal of Emerging Diseases, 8, 598.
- Almansa, J.C. and R.C. Garcia, 1980: Incidencia del murciélago hematófago Desmodus rotundus sobre los indígenas Yanomami de Venezuela, Donana, Acta Vediebrata, 7, 1113–1117.
- Amerasinghe, F.P., 2003: Irrigation and mosquito-borne diseases. Journal of Parasitology, 89 (Suppl.), S14-S22.
- Amerasinghe, P.H., F.P. Amerasinghe, R.A. Wirtz, N.G. Indrajith,W. Somapala, L.R. Pereira, and A.M.S. Rathnayake, 1992: Malaria transmission by indoor resting Anopheles subpictus Grassi in a new irrigation project in Sri Lanka. Journal of Medical Entomology, 29, 577–581.
- Anderson, R.P., D. Lew, and A.T. Peterson, 2003: Evaluating predictive models of species’ distributions: criteria for selecting optimal models. Ecological Modelling 162: 211–232.
- Appawu, M.A., S.K. Dadzie, A. Baffoe-Wilmot, and M.D. Wilson, 2001: Lymphatic filariasis in Ghana: entomological investigation of transmission dynamics and intensity in communities served by irrigation systems in the Upper East Region of Ghana. Tropical Medicine & International Health 6: 511–516.
- Baker, R. 1989: Unpublished report to OCP, Entomological Investigations in Sierra Leone, April–August 1988.
- Baker, R., P. Guillet, A. Seketeli, P. Poudiougo, D. Boakye, M.D. Wilson, and Y. Bissan, 1990: Progress in controlling the reinvasion of windborne vectors into the western area of the onchocerciasis control program in West-Africa. Philosophical Transactions of the Royal Society of London Series B-Biological Sciences, 328, 731–750.
- Beck, L.R., B.M. Lobitz, and B.L. Wood, 2000: Remote sensing and human health: new sensors and new opportunities. Emerging Infectious Diseases, Calif State Univ, Monterey Bay, CA USA NASA, Ames Res Ctr, Moffett Field, CA 94035 USA, 6, 217–227.
- Bell, D., S. Robertson, P.R. Hunter, 2004: Animal origins of SARS corona virus: possible links with international trade in small carnivores. Philosophical Transactions of the Royal Society of London, Biological Sciences. July, 359 (1447), 1107–1114.
- Brieman, R.F., M.R. Evans, W. Preiser, J. Maguire, A. Schnur, A. Li, H. Bekedam, J.S. MacKenzie, 2003: Role of china in the quest to define and control acute respiratory syndrome. Emerging Infectious Diseases; 9 (9), p 1037.
- Ben Mohamed, A. and J.P. Frangi, 1986: Results from ground-based monitoring of spectral aerosol optical-thickness and horizontal extinction—some specific characteristics of dusty Sahelian atmospheres. Journal of Climate and Applied Meteorology, 25, 1807–1815.
- Benton, B., J. Bump, A. Seketeli, and B. Liese, 2002: Partnership and promise: evolution of the African river- blindness campaigns. Annals of Tropical Medicine and Parasitology, 96, 5–14.
- Bielders, C.L., S. Alvey, and N. Cronyn, 2001: Wind erosion: The perspective of grass-roots communities in the Sahel. Land Degradation & Development, 12, 57–70.
- Boakye, D.A., C. Back, G.K. Fiasorgbor, A.P.P. Sib, and Y. Coulibaly, 1998: Sibling species distributions of the Simulium damnosum complex in the West African Onchocerciasis control Programme area during the decade 1984–93, following intensive larviciding since 1974. Medical and Veterinary Entomology, 12, 345–358.
- Borges, E. C., H. H. R. Pires, S. E. Barbosa, C. M. S. Nunes, M. H. Pereira, A. J. Romanha and L. Diotaiuti, 1999: Genetic variability in brazilian triatomines and the risk of domiciliation. Memórias do Instituto Oswaldo Cruz, 94 (Suppl. 1), 371–373.
- Bradley, D., 1977: The health implications of irrigation schemes and manmade lakes in tropical environments.Water, Wastes and Health in Hot Climates. R. Feachem, M. Mcgarry and D. Mara (eds.), John Wiley, London, pp. 18–29.
- Bradley, D. and R. Narayan, 1988: Epidemiological patterns associated with agricultural activities in the tropics with special reference to vector-borne diseases. Effects of Agricultural Development on Vector-Borne Diseases. FAO Publication, No. AGL/MISC/12/87, pp.35–43.
- Brault, A.C., A.M. Powers, D. Ortiz, J.G. Estrada-Franco, R. Navarro-Lopez, S.C. Weaver, 2004: Venezuelan equine encephalitis emergence: enhanced vector infection from a single amino acid substitution in the envelope glycoprotein. PNAS, USA, 101(31), 11344–11349.
- Bunnag, T., S. Sornmani, S. Phinichpongse, C. Harinasuta, 1979: Surveillance of water-borne parasitic infections and studies on the impact of ecological changes on vector mosquitoes of malaria after dam construction. SEAMEO-TROPMED Seminar: environmental impact on human health in Southeast and East Asia, 21st. Tokyo: Tsukuba.
- Busvine, J. R., 1970: Chagas disease control and the possibilities of resistance in triatomids. Observations on a Visit to South America. A World Health Organization Report.
- Busani L., C. Graziani, A. Battisti, A. Franco, A. Ricci, et al., 2004: Antibiotic resistance in Salmonella enterica serotypes Typhimurium, Enteritidis and Infantis from human infections, foodstuffs and farm animals in Italy. Epidemiol Infect, 132, 245–51.
- Capua, I., and D.J. Alexander, 2004: Human health implications of avian influenza viruses and paramyxoviruses. Eur J Clin Microbiol Infect Dis, 23, 1–6.
- Charlwood, J.D. and W.A. Alecrim, 1989: Capture-recapture studies with the South American malaria vector Anopheles darlingi, Root. Annals of Tropical Medicine and Parasitology, 83(6), 569–576.
- Chivian, E., 2001: Species loss and ecosystem disruption: the implications for human health. Canadian Medical Association Journal, Jan, 164(1), 66–69.
- Chua, K.B., W.J. Bellini, P.A. Rota, B.H. Harcourt, A. Tamin, et al. 2000: Nipah virus: a recently emergent deadly paramyxovirus. Science, 288, 1432– 1435.
- Chua, K.B., B.H. Chua, and C.W. Wang, 2002: Anthropogenic deforestation, El Nino and the emergence of Nipah virus in Malaysia. Malaysian J. Pathol. 24, 15–21.
- Cockburn, J. M., 1972: Laboratory investigations bearing on possible insecticide resistance in traitomid bugs. World Health Organization, WHO/VBC/72. 359 pp: 1–9.
- Coelho, G.E., 1995: Aspectos relacionados às agressões humanas da raiva, no Estado de Roraima, Brasil. Monografia, Univ. Brasília, Faculdade de Ciências da Saúde, Brasília, DF, 29 pp.
- Coluzzi, M., 1984: Heterogeneities of the malaria vectorial system in tropical Africa and their significance in malaria epidemiology and control, Bull World Health Organ., 62(Suppl), 107–13.
- Coluzzi, M., 1994: Malaria and the Afrotropical ecosystems: impact of manmade environmental changes, Parassitologia, 36(1–2), 223–7.
- Coluzzi, M, A. Sabatini, V. Petrarca, M.A. Di Deco, 1979: Chromosomal differentiation and adaptation to human environments in the Anopheles gambiae complex, Trans R Soc Trop Med Hyg, 73(5), 483–97.
- Colwell RR, 1996. Global climate and infectious disease: the cholera paradigm. Science 274: 2025–31.
- Confalonieri, U.E.C., 2000: Environmental Change and Human Health in the Brazilian Amazon. Global Change and Human Health, 1(2), 174–83.
- Confalonieri, U.E.C., 2001: Global environmental change and health in brazil: review the present situation and proposal for indicators for monitoring these effects. In: Human Dimensions of Global Environmental Change, D.J. Hogan and M.T. Tomasquin (eds.). Brazilian Perspectives, Acad. Brasil. Cien., Rio de Janeiro, pp. 43–78.
- Connor, S.J., M.C. Thomson, S.P. Flasse, and A.H. Perryman, 1998: Environmental information systems in malaria risk mapping and epidemic forecasting. Disasters, 22, 39–56.
- Cordellier, R, 1991: The epidemiology of yellow fever in Western Africa. Bull World Health Organ, 69, 73–84.
- Costa, J., 1999: The synanthropic process of chagas disease vectors in Brazil, with special attention to triatoma brasiliensis Neiva, 1911 (Hemiptera, Reduviidae, Triatominae) Population, Genetical, Ecological, and Epidemiological Aspects. Mem órias do Instituto Oswaldo Cruz, 94 (Suppl. 1), 239–241.
- Costa, A.I. and D. Natal, 1998: Geographical distribution of dengue and socioeconomic factors in an urban locality in southeastern Brazil. Rev Saude Publica 32: 232–6.
- Costa, M.B., R.F. Bonito, and A.S. Nishioka, 1993: An outbreak of vampire bat bite in a Brazilian Village. Trop. Med. Parasitol, 44, 1219–1220.
- Coura, J.R., A.C. Junqueira, O. Fernandes et al., 2002: Emerging Chagas Disease in Amazonian Brazil. Trends in Parasitology, 18(4):171–176.
- Craig, M.H., R.W. Snow, and D. le Sueur, 1999: A climate-based distribution model of malaria transmission in sub-Saharan Africa. Parasitology Today, 15, 105–111.
- Cunningham, A.A, P. Daszak, and J.P. Rodríguez. In press. Pathogen pollution: defining a parasitological threat to biodiversity conservation. Journal of Parasitology.
- Daszak, P. and A.A. Cunningham, 1999: Anthropogenic change, biodiversity loss and a new agenda for emerging diseases. Journal of Parasitology, Aug., 85(4), 742–746.
- Daszak, P, A. Cunningham, and AD. Hyatt, 2000: Emerging infectious diseases of wildlife-threats to biodiversity and human health. Science, 287(5452).
- Daszak, P., A.A. Cunningham, and A.D. Hyatt, 2001: Anthropogenic environmental change and the emergence of infectious diseases in wildlife. Acta Tropica, 78(2), 103–116.
- Dégallier, N., A.P.A. Travassos da Rosa, J-P. Hervé, P.F.C. Vasconcelos, J.F.S. Travassos da Rosa, G.C. Sá Filho, F.P. Pinheiro, 1989: Modifications of arbovirus eco-epidemiolgy in Tucurui, Pará, Brazilian Amazonia, related to the construction of a hydroelectric dam. Arbovirus Research in Australia, Proceedings 5th Symposium, Queensland I`nstitute of Medical Research, Brisbane, pp.124– 135.
- de Manzione, N., R.A. Salas, H. Paredes, O. Godoy, L. Rojas, et al., 1998: Venezuelan hemorrhagic fever: clinical and epidemiological studies of 165 cases. Clin. Infect. Dis, 26, 308.
- Diotaiuti, L., E.C. Borges, E.S. Lorosa, R.E. Andrade, F.F.C. Carneiro, O.F. Faria Filho and C.J. Schofield, 1998: Current Transmission of Chagas Disease in The State of Ceará, Brazil. Memórias do Instituto Oswaldo Cruz, 93 (Suppl. II), 66.
- Dujardin, J. P., H. Bermúdez and C. J. Schofield, 1997a: The use of morphometrics in entomological surveillance of sylvatic foci of Triatoma infestans in Bolivia. Acta Tropica, 66, 145–153.
- Dujardin, J. P., H. Bermúdez, C. Casini, C. J. Schofield and M. Tibayrenc. 1997b: Metric differences between sylvatic and domestic Triatoma infestans (Hemiptera: Reduviidae) in Bolivia. Journal of Medical Entomology, 34(5), 544–551.
- Dujardin, J., M. Muñoz. T. Chávez, C. Ponce, J. Moreno and C. J. Schofield, 1998: The Origin of Rhodnius prolixus in Central America. Medical and Veterinary Entomology, 12, 113–115.
- Dzodzomenyo, M., S.K. Dunyo, C.K. Ahorlu, W.Z. Coker, M.A. Appawu, E.M. Pederson, and P.E. Simonsen, 1999: Banocroftian filariasis in an irrigation project community in southern Ghana. Trop. Med. Int. Health, Jan., 4(1), 13–18.
- Engelthaler, D.M., D.G. Mosley, J.E. Cheek, C.E. Levy, K.K. Komatsu, et al., 1999: Climatic and environmental patterns associated with hantavirus pulmonary syndrome, Four Corners region, United States. Emerg Infect Dis 5: 87–94.
- Epstein, P.R., E. Chivian, and K. Frith, 2003: Emerging diseases threaten conservation. Environmental Health Perspectives, Volume 111, Number 10, August 2003: A506-A507.
- Epstein, P.R. 1995: Emerging diseases and ecosystem instability: new threats to public health. American Journal of Public Health, 85(2).
- Fa, J.E. and C.A. Peres, 2001: Game vertebrate extraction in African and Neotropical forests: an intercontinental comparison, in Reynolds, J.D., Mace, G.M., Redford, K.H., and Robinson, J.G., Conservation of exploited species: Cambridge, UK, Cambridge University Press, p. 203–241.
- Feer, F., 1993: The potential for sustainable hunting and rearing of game in tropical forests, in Hladik, C.M., Hladik, A., Linares, O.F., Pagezy, H., Semple, A., and Hadley, M. (eds.), Tropical Forests: People and Food: biocultural interactions and applications to development: Pearl River, N.Y., Parthenon Publishing Group, p. 691–708.
- Ferguson, N.M., C. Fraser, C.A. Donnelly, A.C. Ghani, and R.M. Anderson, 2004: Public health risk from the avian H5N1 influenza epidemic. Science, 304, 968–9.
- Field, H., P. Young, J.M. Yob, J. Mills, L. Hall, and J. Mackenzie, 2001: The natural history of Hendra & Nipah viruses. Microbes and Infection, 3, 307–314.
- Gabaldon, A., 1983: Malaria eradication in Venezuela: doctrine, practice, and achievements after twenty years. Am. J. Trop. Med. Hyg., 32, 203–211.
- Gilliver, M.A., M. Bennett, M. Begon, S.M. Hazel, and A. Hart, 1999: Antibiotic resistance found in wild rodents. Nature, 401, 233–234.
- Glass G., J. Cheek, J.A. Patz, T.M. Shields, T.J. Doyle, et al., 2000: Predicting high risk areas for Hantavirus Pulmonary Syndrome with remotely sensed data: the Four Corners outbreak, 1993. J Emerg Infect Dis 2000;6: 239–246.
- Gorla, D. E., J.P. Dujardin, and C.J. Schofield, 1997: Biosystematics of old world triatominae. Acta Tropica, 63, 127–140.
- Graczyk, T.K., B.M. Evans, C.J. Shiff, H.J. Karreman, and J.A. Patz, 2000: Environmental and geographical factors contributing to watershed contamination with cryptosporidium parvum oocysts. Environ. Res., Mar., 82(3), 263–271.
- Graczyk, T.K., A.J. DaSilva, M.R. Cranfield, J.B. Nizeyi, G.R. Kalema, N.J. Pieniazek. 2001: Cryptosporidium parvum genotype 2 infections in freeranging mountain gorillas (Gorilla gorilla beringei) of the Bwindi Impenetrable National Park, Uganda. Parasitol Res, 87, 368–70.
- Gratz, N.G., 1987: The impact of rice production on vector-borne disease problems in developing countries. In: Vector-Borne Disease Control through Rice Agroecosystem Management. International Rice Research Institute, Los Banos, Philippines, pp. 7–12.
- Guerra, J.A., M.L. Barros, N.F. Fe, M.V. Guerra, E. Castellon, M.G. Paes, and I.A. Sherlock, 2004: Visceral leishmaniasis among Indians of the State of Roraima, Brazil: clinical and epidemiologic aspects of the cases observed from 1989 to 1993. Rev Soc Bras Med Trop 37: 305–11.
- Hahn, B.H., G.M. Shaw, K.M. de Cock, and P.M. Sharp, 2000: Aids as a zoonosis: Scientific and public health implications. Science, 287, 607–614.
- Hart, W.S., M.W. Heuzenroeder, and M.D. Barton, 2004: Antimicrobial resistance in Campylobacter spp., Escherichia coli and enterococci associated with pigs in Australia. J Vet Med B Infect Dis Vet Public Health 51, 216–21.
- Hay, S. I., 2000: An overview of remote sensing and geodesy for epidemiology and public health application. Advances in Parasitology, 47, 1–35.
- Hoke, C.H. and J.B. Gingrich, 1994: Japanese Encephalitis. In: Handbook of Zoonoses. 2nd Edition. Section B: Viral. G.W. Beran (ed.). CRC Press, Boca Raton, pp. 59–70.
- Hunter, J.M., L. Rey, K.Y. Chu, E.O. Adekolu-John, and K.E. Mott, 1993: Parasitic diseases in water resources development: the need for intersectoral negotiation. World Health Organization, Geneva, 152 pp.
- Ijumba, J.N. and S. Lindsay, 2001: Impact of irrigation on malaria in Africa: paddies paradox. Medical and Veterinary Entomology, 15, 1–11.
- Institute of Medicine, 2003: Microbial Threats to Health: Emergence, Detection, and Response. National Academy Press, Washington.
- Jewsbury, J.M. and A.M.A. Imevbore, 1988: Small dam health statistics. Parasitology Today, 4, 57–58.
- Jezek Z., I. Arita, M. Mutombo, C. Dunn, J.H. Nakano, and M. Szczeniowski, 1986: Four generations of probable person-to-person transmission of human monkeypox. Am J Epidemiol 123: 1004–12.
- Jobin, W., 1999: Dams and Disease: Ecological design and health impacts of large dams, canals and irrigation systems. E. & Fn. Spon. (Taylor & Francis Group), London & New York, 580 pp.
- Jones, C.G., R.S. Ostfeld, M.P. Richard, E.M. Schauber, and J.O.Wolff, 1998: Chain reactions linking acorns to gypsy moth outbreaks and Lyme disease risk. Science, 279, 1023–1026.
- Joshi, D.D., 1986: Japanese encephalitis in Nepal. JE HFRS Bulletin, 3:15.
- Jouan, A., Adam F, Coulibaly I, Riou O, Philippe B, et al., 1990: Epidemic of Rift Valley fever in the Islamic republic of Mauritania. Geographic and ecological data. Bull. Soc. Pathol. Exot., 83(5), 611–620.
- Kalliola, R, and S. Flores Paitan, eds., 1998: Geoecologia y Desarollo Amazonico: Estudio integrado en la zona de Iquitos, Peru. Sulkava: Finnreklama Oy.
- Kobasa, D., A. Takada, K. Shinya, M. Hatta, P. Halfmann, et al., 2004: Enhanced virulence of influenza A viruses with the haemagglutinin of the 1918 pandemic virus. Nature, 431, 703–7.
- Krause, R.M., 1981: The Restless Tide: The Persistent Challenge of the Microbial World. National Foundation for Infectious Diseases. Washington, DC.
- Kuno G., and R.E. Bailey, 1994: Cytokine responses to dengue infection among Puerto Rican patients. Mem Inst Oswaldo Cruz 89: 179–82.
- Lacey, L.A. and C.M. Lacey, 1990: The medical importance of riceland mosquitoes and their control using alternatives to chemical insecticides. Journal of the American Mosquito Control Association, 6(Suppl):1–93.
- LeChevallier, M.S., and W.D. Norton, 1995: Giardia and Cryptosporidium in raw and finished water. J. Am. Water Works Assoc., 87, 54–68.
- Lederberg, J., R.E. Shope, and S.C. Oakes, 1992: Emerging Infections: Microbial Threats to Health in the United States. Institute of Medicine, National Academy Press. Washington, DC.
- Lefevre, P.C., 1997: Current status of rift valley fever. What lessons to deduce from the epidemics of 1977 and 1987?. Med. Trop., 57(Suppl. 3), 61–64.
- Le Guenno B., P. Formentry, M. Wyers, P. Gounon, F.Walker, and C. Boesch, 1995: Isolation and partial characterisation of a new strain of Ebola virus. Lancet 345: 1271–4.
- Linthicum, K.J., A. Anyamba, C.J. Tucker, P.W. Kelley, M.F. Myers, and C.J. Peters, 1999: Climate and satellite indicators to forecast Rift Valley fever epidemics in Kenya. Science, 285, 397–400.
- Llewelyn, C. A., Hewitt, P. E., Knight, R. S. G., Amar, K., Cousens, S., Mackenzie, J., Will, R. G., 2004: Possible transmission of variant Creutzfeldt- Jakob disease by blood transfusion. The Lancet, 363(9407), 417–21.
- Lobitz, B., L. Beck, A. Huq, B. Wood, G. Fuchs, A.S. Faruque, and R. Colwell, 2000: Climate and infectious disease: use of remote sensing for detection of Vibrio cholerae by indirect measurement. Proc Natl Acad Sci USA, 97, 1438–43.
- Lopez, A. et al., 1992: Outbreak of human rabies in the Peruvian jungle. Lancet, 339, 1408–1411.
- Loutan L. and J.M. Lamotte, 1984: Seasonal variation in nutrition among a group of nomadic pastoralists in Niger. The Lancet 8383 (1), 845 – 947
- MacCarthy, T., 1989: Human depredation by vampire bats (Desmodus rotundus) following a hog cholera campaign. Am. J. Trop. Med. Hyg., 40, 1320–1322.
- Maiztegui, J.I., 1975: Clinical and epidemiologic patterns of Argentine haemorrhagic fever. Bull. WHO, 50:567–575.
- Mak, J.W., 1987: Epidemiology of lymphatic filariasis. Ciba Found Symp, 127, 5–14.
- Marques, A.C., 1986: Migrations and the dissemination of malaria in Brazil. Mem. Inst. Oswaldo Cruz, 81(Suppl II), 17–30.
- Marques, A.C., 1987: Human migration and the spread of malaria in Brazil. Parasitol Today, 3, 166–70.
- Mather, T.H. and T.T. That, 1984: Environmental management for vector control in rice fields. FAO Irrigation and Drainage Paper No. 41, 152 pp.
- Molesworth, A.M., L.E. Cuevas, A.P. Morse, J.R. Herman, and M.C. Thomson, 2002: Dust clouds and spread of infection. Lancet, 359, 81–82.
- Molesworth, A.M., L.E. Cuevas, S.J. Connor, A.P. Morse, and M.C. Thomson, 2003: Environmental risk and meningitis epidemics in Africa. Emerging Infectious Diseases, 9(10), 1287–1293.
- Molyneux, D.H., 1997: Patterns of change in vector-borne diseases. Ann Trop Med Parasitol 91:827–39.
- Monath, T.P., 1994: Dengue: the risk to developed and developing countries. Proc Natl Acad Sci USA 91: 2395–400.
- Moreno, J., Y. Rubio-Palis, and P. Acevedo, 2000: Identificación de criaderos de Anopheles en un área endémica del estado Bolívar. Bol. Dir. Malariol. San. Amb., 40,122–129.
- Moreno, J. and Y. Rubio-Palis, E. Pérez, V. Sánchez, and E. Páez, 2002: Evaluación de tres métodos de captura de anofelinos en un área endémica del estado Bolívar, Venezuela. Entomotropica, 17(2), 157–165.
- Morse, S.S. 1993: Examining the origins of emerging viruses. In: Emerging Viruses. S. S. Morse (ed.) Oxford University Press, New York, pp. 10–28.
- Mutero, C.M., 2002: Health impact assessment of increased irrigation in the Tana River Basin, Kenya. In: The Changing Face of Irrigation in Kenya. Opportu- nities for Anticipating Change in Eastern and Southern Africa. International Water Management Institute (IWMI), Colombo.
- Mutero, C.M., C. Kabutha, V. Kimani, L. Kabuage, G. Gitau, et al. 2003: A transdisciplinary perspective on the links between malaria and agroecosystems in Kenya, Acta Tropica 89: 171–186
- Niamir-Fuller, M. and M.D. Turner, 1999: A review of recent literature on pastoalism and transhumance in Africa. In ‘Managing Mobility in Africa Rangelands— The legitimization of transhumance’ (Niamir-Fuller M., Ed.), pp. 18–46, Intermediate Technology Publications Ltd, London UK.
- Nicholson, S.E., C.J. Tucker, and M.B. Ba, 1998: Desertification, drought, and surface vegetation: An example from the West African Sahel. Bulletin of the American Meteorological Society, 79, 815–829.
- Nizeyi, J.B., R. Mwebe, A. Nanteza, M.R. Cranfield, G.R. Kalema, and T.K. Graczyk, 1999: Cryptosporidium sp. and Giardia sp. infections in mountain gorillas (Gorilla gorilla beringei) of the Bwindi Impenetrable National Park, Uganda. J Parasitol 85: 1084–8.
- Ostfeld, S.R. and F. Keesing, 2000a: Biodiversity and disease risk: the case of Lyme disease. Conservation Biology, 14(3), 722–728.
- Ostfeld, S.R. and F. Keesing, 2000b: The function of biodiversity in the ecology of vector-borne zoonotic diseases. Can. J. Zool., 78, 2061–2078.
- Panzera, F., S. Hornos, J. Pereira, R. Cestau, D. Canale, L. Diotaiuti, J. P. Dujardin and R. Pérez, 1997: Genetic variability and geographic differentiation among three species of triatomine bugs (Hemiptera-Reduviidae). American Journal of Tropical Medicine and Hygiene, 57(6), 732–739.
- Pappagianis D., 1994: Marked increase in cases of coccidioidomycosis in California: 1991, 1992, and 1993. Clin Infect Dis 19 Suppl 1:S14-S18.
- Parrish, C.R., P.H. O’Connell, J.F. Evermann, and L.E. Carmichael, 1985: Natural variation in canine parvovirus. Science, 230,1046–1048.
- Parmenter, C.A., T.L. Yates, R.R. Parmenter, and J.L. Dunnum, 1999: Statistical sensitivity for detection of spatial and temporal patterns in rodent population densities. Emerg Infect Dis 5: 118–25.
- Patz, J.A., T.K. Graczyk, N. Geller, and A.Y. Vittor. 2000: Effects of environmental change on emerging parasitic diseases. Int J Parasitol, 30, 1395–1405.
- Patz, J.A., P. Daszak, G.M. Tabor, A.A. Aguirre, M. Pearl, et al., 2004: Unhealthy Landscapes: Policy Recommendations on Land Use Change and Infectious Disease Emergence. Environ Health Perspect, 101:1092–98.
- Peiris, J.S.M., F.P. Amerasinghe, P.H. Amerasinghe, C.B. Ratnayake, S.H.P.P. Karunaratne, and T.F. Tsai, 1992: Japanese encephalitis in Sri Lanka—the study of an epidemic: vector incrimination, porcine infection and human disease. Transactions of the Royal Society of Tropical Medicine and Hygiene, 86, 307–313.
- Peterson, A.T., V. Sanchez-Cordero, C.B. Beard, and J.M. Ramsey, 2002: Ecologic niche modeling and potential reservoirs for Chagas disease, Mexico. Emerg Infect Dis 8: 662–7.
- Peterson, A.T., R.S. Pereira, and V.F. Neves, 2004a: Using epidemiological survey data to infer geographic distributions of leishmaniasis vector species. Rev Soc Bras Med Trop 37: 10–4.
- Peterson, A.T., J.T. Bauer, and J.N. Mills, 2004b: Ecologic and geographic distribution of filovirus disease. Emerg Infect Dis 10: 40–7.
- Pinheiro, F.P., G. Bensabath, A.P.A. Travassos da Rosa, R. Lainson, J.J. Shaw, et al. 1977: Public health hazards among works along the Trans-Amazon Highway. J. Occupat. Med., 7, 490–497.
- Pinheiro, F.P., A.P.A. Travassos da Rosa & P.F.C. Vasconcelos, 1998: An overview of Oropouche fever epidemics in Brazil and neighbour countries. In: Travassos da Rosa, A.P.A., P.F.C. Vasconcelos & J.F.S. Travassos da Rosa (ed.). An overview of arbovirology in Brazil and neighbouring countries. Instituto Evandro Chagas, Bel ém, pp.186–192.
- Povoa, M.M., J.E. Conn, C.D. Schlichting, J.C.Amaral, M.N. Segura, et al., 2003: Malaria vectors and the re-emergence of Anopheles darlingi in Belém, Par á, Brazil. J. Med. Enotmol. Jul; 40 (4): 379–86.
- Povoa, M., R. Wirtz, R. Lacerda, M. Miles, D. Warhurst, 2001: Malaria vectors in the municipality of Serra do Navio, State of Amapa, Amazon Region, Brazil. Mem Inst Oswaldo Cruz, Feb;96(2), 179–84.
- Prospero, J.M., 2001: African dust in America. Geotimes, 46, 24–27.
- Prusiner, S.B., 1997: Prion diseases and the BSE crisis. Science, 278, 245–51.
- Rabinovich J., N. Schweigmann, V. Yohai, and C. Wisnivesky-Colli, 2001: Probability of Trypanosoma cruzi transmission by Triatoma infestans (Hemiptera: Reduviidae) to the opossum Didelphis albiventris (Marsupialia: Didelphidae). Am J Trop Med Hyg 65: 125–30.
- Ramasamy, R., R. de Alwis, A. Wijesundera, and M.S. Ramasamy, 1992: Malaria transmission at a new irrigation project in Sri Lanka: the emergence of Anopheles annularis as a major vector. American Journal of Tropical Medicine and Hygiene, 47, 547–553.
- Roberts, L., U.E.C. Confalonieri, and J.L. Aaron, 2001: Too Little, Too Much: How the Quantity of Water Affects Human Health. Chapter in, Aaron J.L. and Patz J.A. (eds), Ecosystem Change and Public Health: A Global Perspective. Johns Hopkins University Press, Baltimore.
- Rodo, X., M. Pascual, G. Fuchs, A.S.G. Faruque, 2002: ENSO and cholera: a nonstationary link related to climate change? Proc. Natl. Acad Sci. USA. Oct, 99(20), 12901–6.
- Rose, J.B., D.E. Huffman, and A. Gennaccaro, 2002: Risk and control of waterborne cryptosporidiosis. FEMS Microbiol Rev 26: 113–23.
- Saeed, M.F., H. Wang, M. Nunes, P.F.C. Vasconcelos, S.C. Weaver, R.E. Shope, D.M. Watts, R.B. Tesh, A.D.T. Barrett, 2000: Nucleotide sequences and phylogeny of the nucleocapsid gene of Oropouche virus. J. Gen. Virol., 81, 743–748.
- Salas, R., N. de Manzione, R.B. Tesh, R. Rico-Hesse, R.E. Shope, A. Betancourt, O. Godoy, R. Bruzual, M.E. Pacheco, B. Ramos, et al., 1991: Venezuelan haemorrhagic fever. Lancet, 338 (8774) 1033–6.
- Schmidt, K.A. and R.S. Ostfeld, 2001: Biodiversity and the dilution effect in disease ecology. Ecology, 82(3), 609–619.
- Schneider, M.C, 1991: Situación Epidemiológica de la Rabia Humana Transmitida por Murciélagos en el Brasil. In: Reunión de Consulta sobre la Atención a Personas Expuestas a la Rabia Transmitida por Vampiros, Washington, DC, Abril 1–5, 1991. Organización Panamericana De La Salud, pp. 63–82.
- Schneider, M.C. and C.S. Burgoa, 1995: Algunas consideraciones sobre la rabia humana transmitida por murciélago. México, Rev. Salud. Publ., 37, 354–362.
- Schneider, M.C., C. Santos-Burgoa, J. Aron, B. Munoz, S. Ruiz-Velazco, and W. Uieda, 1996: Potential force of infection of human rabies transmitted by vampire bats in the Amazon region of Brazil. Am. J. Trop. Med. Hyg., 55(6), 680–684.
- Schneider E., R.A. Hajjeh, R.A. Spiegel, R.W. Jibson, E.L. Harp, et al. 1997: A coccidioidomycosis outbreak following the Northridge, Calif, earthquake. Jama-Journal of the American Medical Association 277: 904–908.
- Schofield, C. J., 1994: Triatominae, Biology and Control. Eurocommunica Publications, UK.
- Schofield, C. J. and J. P. Dujardin. 1997: Chagas disease vector control in Central America. Parasitology Today 13(4), 141–144.
- Service, M.W., 1984: Problems of vector-borne diseases and irrigation projects. Insect Science and its Application, 5, 227–231.
- Service, M.W., 1989: Rice, a challenge to health. Parasitology Today, 5, 162– 165.
- Sharma, V.P., 1996: Re-emergence of malaria in India. Indian Journal of Medical Research, 103, 26–45.
- Sheik-Mohamed, A. and J.P. Velema, 1999: Where health care has no access: the nomadic populations of sub-Saharan Africa. Tropical Medicine and International Health; 4(10), 695–707.
- Shiraki, Y., N. Shibata, Y. Doi, and Y. Arakawa, 2004: Escherichia coli producing CTX-M-2 beta-lactamase in cattle, Japan. Emerg Infect Dis 10, 69–75.
- Shope, R.E., 1997: Emergence of arbovirus diseases following ecological modifications: epidemiological consequences. In: Factors in the emergence of Arbovirus diseases, Saluzzo JF, B. Dodet (ed.). Elsevier, Paris, pp.19–22.
- Silveira, F.T, J.J. Shaw, C.N. Bichara, et al., 1997: Leishamniose Tegumentar Americana, in R.N.Q. Leão (org.), Doenças Infecciosas e Parasitárias. Enfoque Amazônico. CEJUP, Belém, Pará, pp. 631–44.
- Simpson, D.I.H., 1978: Viral haemorrhagic fevers of man. Bull. WHO, 56(6), 819–832.
- Sioli H., 1953: Schistosomiasis and limnology in the Amazon region. Am J Trop Med Hyg 2: 700–7.
- Southgate, V.R., 1997: Shistosomiasis in the Senegal River Basin: before and after the construction of dams at Diama, Senegal and Manantali, Mali and future prospects. J Helminthol, 71(2), 125–32.
- Souza, J.M., 1995: Malaria autóctone na grande Belém: problema dos poderes constituídos (Estado e Município da Grande Belém), ao lado das empresas e associac¸ões comunitárias das áreas atingidas. O Plasmodio, 2:1–2.
- Stark, G., 2003: ‘‘Causes and consequences and control of wind erosion in Sahelian Africa: a review.’’ Land Degradation & Development 14: 95–108.
- Steindel, M., 1999: Trypanosoma cruzi interaction with its vectors and vertebrate hosts. Memórias do Instituto Oswaldo Cruz, 94 (Suppl. 1), 243–245.
- Sunish I.P. and R. Reuben, 2001: Factors influencing the abundance of Japanese encephalitis vectors in ricefields in India—I. Abiotic. Medical and Veterinary Entomology 15: 381–392.
- Surtees, G., 1975: Mosquitoes, arboviruses and vertebrates. In: Man-made lakes and human health, N.F. Stanley and M.P. Alpers (eds.). London Academic Press, pp. 21–34.
- Tadei, W.P., B.T. Thatcher, J.M.M. Santos, V.M. Scarpassa, I.B. Rodríguez, and M.S. Rafael, 1998: Ecologic observations on anopheline vectors of malaria in the Brazilian Amazon. Am. J. Trop. Med. Hyg., 59, 325–335.
- Taylor, L.H., S.M. Latham, and M.E.J. Woolhouse, 2001: Risk factors for human disease emergence. Phil. Trans. Soc. Lond. B., 356, 983–989.
- Teixeira, A.R.L., P.S. Monteiro, J.M. Rebelo et al., 2000: Emerging Chagas Disease: trophic network and cycle of transmission of Trypanosoma cruzi from palm trees in the Amazon. Emerging Infectious Diseases 7(1):100–112.
- Thomas, J.G. and H.J. Haran, 1981: Vampire bat bites seen in humans in Panamá: their characterization, recognition and management. Milit. Med., 146, 410–412.
- Thomson, M.C. and S.J. Connor, 2000: Environmental information systems for the control of arthropod vectors of disease. Medical and Veterinary Entomology, 14, 227–244.
- Thomson, M.C. J. B. Davies, R. J. Post, M. J. Bockarie, P. A. BeechGarwood, and J. Kandeh, 1996: The unusual occurrence of savanna members of the Simulium damnosum species complex (Diptera: Simuliidae) in southern Sierra Leone in 1988. Bulletin of Entomological Research, 86,(3) 271–280.
- Thomson, M.C., D.A. Elnaiem, R.W. Ashford, and S. J. Connor, 1999: Towards a kala azar risk map for Sudan: mapping the potential distribution of Phlebotomus orientalis using digital data of environmental variables. Trop Med Int Health, 4, 105–13.
- Tyagi, B.K., 2002: Malaria in the Thar Desert: facts, figures and future. Agrobis, Índia, 165 pp.
- Tyssul Jones, T.W., 1951: Deforestation and epidemic malaria in the wet and intermediate zones of Ceylon. Indian J Malariology, 5(1), 135–61.
- Uieda, W., G.E. Coelho, and I. Menegola, 1992: Morcegos Hematófagos IV. Agressões humanas no Estado de Roraima e considerações sobre sua incidência na Amazônia. Resumo, Seminário Nacional de Raiva, S. Paulo, 7 – 11 Dez, 1992, Inst. Biológico, S. Paulo (mimeo).
- UNEP (United Nations Environment Programme) (in press). Sustaining Life: How Human Health Depends on Biodiversity. Oxford University Press – Interim executive summary available at: www.med.harvard.edu/chge/bio .html.
- Vasconcelos, P.F.C., A.P.A. Travassos da Rosa, S.G. Rodrigues, E.S. Travassos da Rosa, N. Dégallier, J.F.S. Travassos da Rosa, 2001: Inadequate management of natural ecosystem in the Brazilian Amazon region results in the emergence and reemergence of arboviruses. Cadernos de Saúde Pública, 17(Supl.), 155–164.
- Victor, T.J. and R. Reuben, 2000: Effects of organic and inorganic and inorganic fertilizers on mosquito populations in rice fields of southern India. Med Vet Entomol., 14, 361–368.
- Vittor A.Y., R. Gilman, J. Tielsch, G.E. Glass, T.M. Shields, W.S. Lozano, V.P. Cancino, and J.A. Patz, (in press): The impact of deforestation on malaria risk: association between human-biting rate of the major South American malaria vector, Anopheles darlingi, and the extent of deforestation in the Peruvian Amazon. J Am Trop Med Hyg.
- Walsh, J.F., D.H. Molyneux, and M.H. Birley, 1993: Deforestation: effects on vector-borne disease. Parasitology 106 Suppl: S55–75.
- Watts, D.M., G. Ramirez, C. Cabezas, M.T. Wooster, C. Carrillo, M. Chuy, E.J. Gentrau, C.G. Hays, 1998: Arthropod-borne viral diseases in Peru. In: Travassos da Rosa, A.P.A., P.F.C. Vasconcelos, J.F.S. Travassos da Rosa (ed.). An overview of arbovirology in Brazil and neighbouring countries. Instituto Evandro Chagas, Belém, pp. 193–218.
- WCD (World Commission on Dams), 2000: Dams and Development: A New Framework for Decision-Making. Earthscan, UK, 404 pp.
- Webster, R.G., 2004:Wet Markets- a continuing source of severe acute repiratory syndrome and influenza. Lancet, 363 (9404); 234.
- WHO (World Health Organization), 1996: Ebola haemorrhagic fever in Gabon—Update 3 from 26 April 1996. [online] World Health Organization, Geneva. Available at http://www.who.int/csr.don/1996_04_26b/en/.
- WHO, 2001: Malaria Early Warning Systems, Concepts, Indicators and Partners, ‘‘A framework for field research in Africa’’. WHO, Geneva.
- WHO, 2002: World Health Report, 2002: Reducing Risks, Promoting Healthy Life. WHO, Geneva.
- WHO, 2003: Summary of probable SARS cases with onset of illness from 1 November 2002 to 31 July 2003. [online] World Health Organization, Geneva. Cited September 26, 2003. Available at http://www.who.int/csr/sars/ country/table2003_09_23/en/.
- WHO, 2004: World Health Report, 2004: Changing History. WHO, Geneva.
- WHO/TDR, 2002: Disease burden and epidemiological trends. [online] World Health Organization. Cited February 2002. Available at http://www.who .int/tdr/diseases/lymphfil/direction.htmburden.
- Wijesundera, M. de S., 1988: Malaria outbreaks in new foci in Sri Lanka. Parasitology Today, 4, 147–150.
- Wilson, M.D., et al., 2002: Deforestation and the spatio-temporal distribution of savannah and forest members of the Simulium damnosum complex in southern Ghana and south-western Togo. Transaction of The Royal Society of Tropical Medicine and Hygiene, 96, 632–639.
- Wolfe, N.D., A.A. Escalante, W.B. Karesh, A. Kilbourn, A. Spielman, A.A. Lal, 1998: Wild primate populations in emerging infectious disease research: The missing link? Emerging Infectious Diseases, Apr-Jun, 4(2) 149–158.
- Wolfe, N.D., M.N. Eitel, J. Gockowski, P.K. Muchaal, C. Nolte, A.T. Prosser, J.N. Torimiro, S.F. Weise, D.S. Burke, 2000: Deforestation, hunting and the ecology of microbial emergence. Global Change and Human Health, 1(1), 10–25.
- Wolfe N.D., W.M. Switzer, J.K. Carr, V.B. Bhullar, V. Shanmugam, et al., 2004: Naturally acquired simian retrovirus infections in central African hunters. Lancet 363:932–7(2004).
- Woods, C.W., A.M. Karpati, T. Grein, N. McCarthy, P. Gaturuku, et al., 2002: An Outbreak of Rift Valley Fever in Northeastern Kenya, 1997–98. Emerging Infectious Diseases, Feb:8 (2).
Terms of Use
The copyright for material on this page is the property of the World Resources Institute. Click here for the Terms of Use (Ecosystems and Human Well-Being: Volume 1: Current State and Trends: Human Infectious Disease Agents).
Disclaimer: This chapter is taken wholly from, or contains information that was originally written for the Millennium Ecosystem Assessment as published by the World Resources Institute. The content has not been modified by the Encyclopedia of Earth.
|
|