Disappearing Jewels: Chapter 3
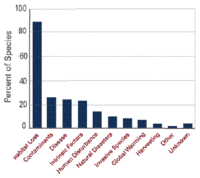 Figure 19. Comparison of risk factors affecting threatened amphibians. (Source: NatureServe)
Figure 19. Comparison of risk factors affecting threatened amphibians. (Source: NatureServe) What is causing so many amphibian species throughout the New World to be threatened? Two major factors appear to be at play. First, the loss of suitable quality habitat is clearly the reason most species enter into one of the threat categories. Fully 89% of all threatened species suffer from habitat loss (Figure 19). The most important factors causing habitat loss include conversion to agriculture, deforestation, mining, development of infrastructure, including housing, industry, roads, and dams (Figure 20). Fires also degrade habitat for a number of species.
Focusing on the Critically Endangered species shows that a second factor is causing the most rapid declines and disappearances of species, outpacing habitat destruction in many cases. In 158 (47%) of the Critically Endangered species, population declines in the absence of habitat loss are linked to or show patterns associated with the effects of a newly identified fungal disease, possibly working in concert with climate change. These species tend to inhabit mid to high elevations, are often associated with streams, and tend to disappear without any obvious destruction to their habitats[1]. The pattern is more pronounced in the “possibly extinct” species (those with no known populations, but lacking sufficient searching to verify extinction), of which 74 species (65%) have or may have been affected by disease.
About one-fourth of all threatened species are threatened by environmental contaminants, disease, and intrinsic factors such as small range size or limited ability to disperse. Human disturbance, including tourist activities, fires, and armed conflicts, affects 13% of threatened species. Natural disasters (such as droughts, floods, and wildfires), alien species, and changes in climate each affect about eight percent of species. Harvesting for consumption, the pet trade, or scientific research is a cause of decline for just three percent of threatened species.
For one set of risk factors, including habitat destruction, invasive species, and harvesting, determining that they cause amphibian declines is a straightforward process. When forests are cut down, forest-dwelling species will decline sharply or disappear. When collectors export thousands of frogs, native populations suffer. The ecological basis for these processes is well understood. For other risk factors, however, including disease, global warming, and contaminants, scientists are much more challenged to demonstrating cause and effect[2]. A diseased frog may crawl under a rock to die and never be seen by a scientist. Especially in tropical habitats, dead animals decompose in a matter of hours leaving no trace. Pesticides can blow in on the wind and debilitate animals such that they are more susceptible to disease. Finding pesticide residues in wild animals is a difficult task, and proving that these chemicals were the cause of death is even more challenging. For this reason, statistics on the exact number of species affected by a particular cause will always be approximate, and scientists must look at characteristic patterns of decline to infer cause.
Contents
- 1 Habitat loss
- 2 Disease
- 3 Pesticides
- 4 Climate Change
- 5 Invasive species
- 6 Trade
- 7 Synergistic effects
- 8 Notes This is a chapter from Disappearing Jewels: The Status of New World Amphibians (e-book). Previous: The Status of New World Amphibians (Disappearing Jewels: Chapter 3) |Table of Contents (Disappearing Jewels: Chapter 3)|Next: Conserving Amphibians: An Agenda for the Future
Habitat loss
 Figure 20. Causes of habitat loss affecting threatened amphibians. (Source: NatureServe)
Figure 20. Causes of habitat loss affecting threatened amphibians. (Source: NatureServe) The complex habitat needs of [[amphibian]s] present a special challenge for conservation. Some species are entirely terrestrial. Some are entirely aquatic. Most use both habitat types during different stages of their life cycle, meaning that land managers must protect both natural lands and waters in order to maintain amphibian diversity (Species diversity).
Our growing human population and its demand for food, shelter, energy, and consumer goods continue to drive the destruction of habitats throughout the hemisphere. Although population growth rates are substantially lower than they were in the 1960s and 1970s, most countries are still growing. The United Nations’ mid-range estimate for Latin America and the Caribbean projects that the human population will swell 48% to 768 million people by 2050. Similarly, the UN projects the population of North America to increase 42% to 448 million people over the same period[3]. All of these people will place additional burdens on our natural resources, and amphibians and other wildlife will be pushed further to the margins of their existence.
Forest cover continues to diminish. During the 1990s alone, South America lost four percent of its forest cover and the islands of the Caribbean lost a whopping ten percent. North America had essentially no net change in forest cover during the last decade[4], but this seemingly favorable circumstance masks the loss of old growth and mature forest that was replaced by much younger forests, which generally are much less favorable as amphibian habitat. Our compilation of the causes of habitat loss directly affecting amphibians shows that agriculture, logging, and development are most important. In many areas, logging occurs first, then land is cultivated, and finally development eats up farm land. The details vary with geography. For example, in Chile, native southern beech (genus Nothofagus) forests are being replaced directly with plantations of introduced pines where few amphibians can persist. In Colombia, coca farmers clear patches of forest in a cat and mouse game with authorities. In Honduras, subsistence farmers relentlessly push back the agricultural frontier. In the northwestern United States, pressure persists to fell more old-growth forest. Whatever the process, the result is the same for amphibians.
Wetlands are disappearing, too, but at a rate that is much more difficult to quantify. Although large wetlands are detectable in satellite imagery, the forested swamps, small creeks, springs, and temporary pools that are essential for the reproduction of many amphibians simply do not show up. Thus scientists are challenged to monitor change in the availability of wetlands. In the United States, the Fish and Wildlife Service estimates that wetlands loss has slowed sharply in recent decades to 23,700 hectares per year or 0.05% of the total wetlands area[5]. This is an encouraging sign and a tribute to the power of effective legislation.
In other countries, standardized data on change in the extent of wetlands are virtually nonexistent[6]. Some of the world’s most spectacular wetlands are found in South America, including the Orinoco and Amazon River drainages, the Llanos wetlands in Venezuela and Colombia, the Pantanal of Brazil, and the wet chaco of Paraguay and Argentina. But these natural wonders, together with rivers throughout the hemisphere, are threatened by dams that disrupt natural hydrological regimes. Brazil, for example, obtains 90% of its energy from hydroelectric plants[7], and power companies everywhere are regularly prospecting for new dam sites. Wetlands monitoring and the development of strategies to ameliorate the negative effects of hydroelectric projects on [[amphibian]s] and other aquatic wildlife are priorities for future work.
Disease
Until about 15 years ago, few people who worked with [[amphibian]s] paid much attention to diseases. Zookeepers of course were worried about diseases striking their animals, but field researchers rarely had any reason to suspect diseases were much of a factor in the dynamics of the populations they studied. Dr. John Lynch, the taxonomist who has discovered more new species of amphibians in the world than anyone else, recalled that prior to 1997 he “had never seen more than 2-3 dead or dying frogs in a single field season”[8]. If field researchers did not see dead or dying frogs then how could disease be an important mortality factor?
Although certain natural pathogens have long been known to attack amphibians, none had been implicated in widespread declines. By the 1990s, however, scientists began to suspect that diseases might be playing a role in cases where amphibian populations were crashing in otherwise undisturbed [[habitat]s]. In 1996, Dr. William Laurance and colleagues looked at the pattern of disappearances of 14 species of frogs endemic to Australia’s east coast and concluded that only an emerging, highly pathogenic disease could explain the pattern[9]. By that time, several sites in Costa Rica had lost substantial numbers of species, and some frogs and toads in western North America had inexplicably declined[10][11][12]. Scientists were frustrated because examination of dead frogs revealed no known pathogen that could have caused such widespread mortality.
The mystery was resolved in 1998 when scientists used an electron microscope to examine skin sections of dead Central American and Australian frogs. They found a previously unknown fungus, now named Batrachochytrium dendrobatidis[13][14] (see also [15]). B. dendrobatidis, referred to as Bd, belongs to a group of fungi called chytrids, which were not known to be pathogenic to vertebrates. Chytrids occur naturally in diverse ecosystems and play an important role in digesting proteins such as chitin from insect exoskeletons, cellulose from plants, keratin from hair and skin, and pollen[16][17]. In amphibians, Bd appears to attack keratin in the beaks of tadpoles and the skin of adults. The exact mechanism of death is still unknown. Bd may produce a toxin that kills the host, or perhaps they affect the passage of moisture, nutrients, or contaminants across the permeable skin.
Three aspects of the biology of B. dendrobatidis help explain patterns of amphibian decline. First, this chytrid will grow in culture only in cool temperatures. This may explain why montane species are more likely to decline than lowland species[18]. Second, like most chytrids, Bd appears to occur only in aquatic habitats, which would explain why amphibians that spend at least part of their life cycle near streams are more likely to decline. Third, Bd affects primarily the keratinized beak of tadpoles, explaining why tadpoles in affected areas can be missing their beaks.
These observations raise many questions about the role of chytrid disease in amphibian declines. Have Bd outbreaks been occurring all along, unnoticed until now? Or is it a disease that has only recently spread to much of the world, wiping out native populations that have not evolved defenses against the disease? How does Bd move from place to place? How is the disease transmitted from individual to individual?
In an attempt to answer the question of origin, Erica Morehouse and colleagues looked at the genetic variation of Bd strains isolated from wild amphibian populations in North America, Africa, and Australia. Their DNA analysis suggested that chytrids have recently spread worldwide from a single source[19]. Dr. Patricia Burrowes and colleagues examined 106 museum specimens of frogs collected on the island of Puerto Rico from 1961 to 1978. The earliest they detected chytrids was on a specimen collected in 1976, suggesting a recent arrival of the disease to that island[20]. Inspection of amphibian specimens elsewhere shows that Bd was present in the United States as early as 1974 and in Australia as early as 1978[21][22].
Details about transmission and movement of the disease are still sketchy. Certainly an uninfected animal can become infected when it enters a body of water that has been contaminated by diseased individuals[23]. Outside of the water, it appears that only physical contact between animals can transmit the disease. Long-distance dispersal appears to occur only when infected frogs themselves move, usually with human help. For example, Bd leapfrogged from eastern to western Australia by hitchhiking on a frog that stowed away in a crate of fruit[24].
Does this mean that Bd is to blame for all amphibian declines in protected areas? Possibly, but we may never know for sure. Other factors cited here—pesticide drift, other diseases, and climate change—play a role. Most frogs die without any witnesses around to collect a specimen. For innumerable populations that simply disappeared, we will never be certain of the cause. Nevertheless, we now have enough data to conclude that Bd outbreaks, possibly in concert with changing climates, have contributed significantly to the decline of amphibian populations[25][26][27].
Pesticides
Beginning in the 1950s, technological advances have dramatically changed the way we grow our food crops, increasing yields substantially. A cornerstone of this “green revolution” is the cornucopia of [[pesticide]s] now used by the world’s farmers to control weeds, diseases, and animal pests. Data from the Food and Agriculture Organization (Food and Agriculture Organization (FAO)) of the United Nations (FAO) show that pesticide use in the Americas remained high during the period 1990-2000, averaging several kilograms per hectare of cultivated land[28].
Pesticides vary tremendously in their toxicity and permanence in the environment. Some of the worst in terms of their effects on wildlife have been banned, but many others remain on the market. Rains and [[wind]s] cause these chemicals to be washed or blown into natural [[habitat]s] surrounding agricultural areas where they can harm native species[29][30]. Amphibians, with their permeable skin and aquatic habits, might be particularly susceptible to these chemicals. Amphibians also consume large amounts of insects, including aquatic insects. If prey animals are contaminated with pesticides, then these substances can build up over time in amphibian tissues, leading in some cases to death or malformation (see Box 8).
Information on the effects of pesticides on amphibian populations is limited, but wind- and water-borne pesticides could potentially explain declines in sites where no obvious habitat loss has occurred. One place where the interaction between pesticide use and amphibian populations has been studied is California, one of the most intensively farmed regions of the world. In 1998 alone, California farmers used 90 million kilograms of pesticides[31]. Previous work has shown that winds blow pesticides long distances from where they are applied and that wild amphibians in California can have traces of agrochemicals in their tissues, suggesting a possible causal link. Dr. Carlos Davidson and colleagues analyzed the spatial patterns of pesticide drift and declines in eight amphibian species. In four of these species—the red-legged frog (Rana aurora), Cascades frog (Rana cascadae), foothill yellow-legged frog (Rana boylii), and mountain yellow-legged frog (Rana muscosa)—declines were more likely in populations directly downwind of large agricultural areas than in populations downwind of land with relatively little agricultural activity[32]. This pattern, repeated in different parts of the state and for different frog species, strongly suggests a roll for [[pesticide]s] in amphibian declines in otherwise undisturbed habitat. In Latin America, studies of the effects of contaminants on amphibians are just beginning[33][34], but the volume of pesticides applied in agricultural areas strongly warrants further investigation.
Climate Change
Our climate is changing, and much of this change is due to carbon dioxide and other greenhouse gases released into the atmosphere by human activity[35]. Recent research has shown that climate change is not an abstract possibility, but rather an ongoing event actually happening today with measurable affects on wild organisms. In the north temperate zone, many plants and animals are stretching their ranges northward. The dates on which some birds lay their eggs, butterflies emerge from their cocoons, and alpine wildflowers bloom are happening earlier. Amphibians are crawling out of their overwintering burrows and starting their mating choruses sooner in the spring than at any time in the last century[36][37]. What role might these climate changes play in amphibian population declines and extinctions?
Like contaminants, climate change can act on amphibians in a number of ways. The ranges of many species are determined not only by favorable [[habitat]s], but by a specific set of environmental conditions such as temperature and precipitation. As climates change, the location of these “climate envelopes” moves across the landscape. Organisms adapted to a particular climate envelope have to move with the envelope to avoid extinction. For example, trees and many other organisms of North America moved back and forth, north and south, during the successive ice ages and interglacial warming periods of the past 200,000 years. Problems arise for species restricted to mountaintops or protected areas surrounded by unsuitable habitat. As the temperature warms, the climatic envelope moves away and species are left with nowhere to go. Although there are as yet no definitive cases of this phenomenon leading directly to the extinction of an amphibian species, it most likely will happen in the next 100 years[38]. In fact, by the end of the century climate change may outpace habitat loss as the most important threat to biodiversity[39][40].
The clearest evidence that this process is underway comes from tropical cloud forests, where montane forests are constantly bathed in a cloud-borne mist. Studies have shown that either deforestation or small changes in sea surface temperature upwind can cause the cloud level to rise significantly[41][42]. Animal distributions in at least one Central American cloud forest are already responding to this change[43].
Climate change can also have more direct effects on [[amphibian]s]. A drying trend can mean that temporary pools that some species require for reproduction may dry up before tadpoles have had a chance to complete metamorphosis. Additionally, increased temperatures and/or less precipitation can stress amphibians, leading to greater susceptibility to disease[44]. Thus climate change may also act indirectly by causing local biological changes that increase amphibian mortality.
To draw firm conclusions, we need sites with both comprehensive, long-term climate data as well as long-term population [[monitor]ing] data. Rarely are both available for the same place. Without data pinpointing the timing of declines and showing climatic trends, documenting a relationship between climate and decline events is impossible. Yet in three tropical sites, highland Costa Rica, Andean Ecuador, and montane Puerto Rico, the requisite combination of population and climate data is available for analysis. In the Costa Rican site, 20 species of frogs and toads, including the golden toad (Bufo periglenes), declined or disappeared abruptly in 1988, with subsequent sharp declines of survivors in 1994 and 1998. Each of these decline events occurred during unusual dry periods when the typical cloud-borne mist failed to form[45]. Andean Ecuador was home to the spectacular jambato toad (Atelopus ignescens), which suddenly disappeared from 47 sites in the 1980s, just after the two driest years recorded during the period 1962-1998[46]. Similarly, drought accompanied the disappearance of three species and the decline of six species of frogs from the genus Eleutherodactylus in Puerto Rico[47]. In all of these cases, weather may have interacted with disease to cause the declines (see Synergistic Effects below).
Invasive species
Invasive species brought to foreign lands, whether by intention or accident, are well known to cause extinctions of the native flora and fauna. How have these invasive species affected New World [[amphibian]s]?
Invasive species are threatening amphibians in a number of ways. Introduced trout that prey principally on amphibian larvae have severely depressed populations of mountain yellow-legged frogs in California (see Box 9). A number of western North American pond-breeding salamanders also suffer population reductions from the presence of introduced trout. Trout have colonized mountain streams from Central America through the Andes as a result of both intentional introductions and escapes from fish farms. Comprehensive studies of the effects of trout on tropical stream amphibians have yet to be undertaken so we do not yet know the impact of this introduced predator on amphibian populations.
Bullfrogs (Rana catesbeiana) are native to eastern North America, but have invaded western North America and parts of the Caribbean and South America. Bullfrogs are widely exported to frog farms that sell frog legs for the local restaurant trade. Some animals, defying their intended fate, escape from their tanks and establish feral populations. These voracious eaters can consume prodigious quantities of food, including tadpoles and adults of native species. In some areas, introduced bullfrogs have played a role in the decline of local leopard frog species.
Even innocuous looking annual plants threaten [[amphibian]s]. Purple loosestrife (Lythrum salicaria), native to Europe, has spread widely in eastern North America. Although its lavender flowers brighten up roadsides throughout its new range, purple loosestrife catalyzes the filling in of seasonal and permanent wetlands. As each wetland disappears, so do the spring choruses of the frogs and toads that need healthy wetlands to reproduce.
Trade
One of the remotest places in the New World is the Gran Chaco, a dry, low-lying region at the border of Bolivia, Paraguay, and Argentina. There you can be 150 kilometers from the nearest place to buy a soft drink (farther if you want a cold one). Even here, several species of frogs, including horned frogs (genus Ceratophrys) and tropical bullfrogs (genus Leptodactylus) are starting to miss their annual singing dates that occur with the onset of seasonal rains. The reason? Their odd shapes and coloration (Ceratophrys cranwelli looks like a giant mouth with eyes and legs thrown on for decoration) make them prized for the pet trade. Although dealers in destination countries profit the most, the small amounts of cash paid to local collectors explain why they are willing to brave the elements and distance to collect these frogs.
Overall, trade in [[amphibian]s] for pets, consumption, and scientific use is cited as a factor in the decline of only 36 threatened species (3% of the total). Harvest of frogs and salamanders as a local food source and traditional medicine has affected species such as frogs in the genus Telmatobius in Peru and Bolivia (see Box 6), the salamander Ambystoma dumerili (the skin of which is made into a medicinal syrup for respiratory ailments by nuns in a convent near Lake Pátzcuaro, Mexico, where the species occurs)[48], and the mountain chicken (Leptodactylus fallax), a large frog consumed on Dominica and Montserrat[49]. International trade is regulated under the Convention on International Trade in Endangered Species of Wild Fauna and Flora (CITES), a treaty that has been signed by 160 countries. The existence of a clandestine trade in these amphibians is proven by regular confiscations by customs officials in exporting and importing countries. On the balance, however, commercial trade alone has pushed relatively few amphibians into the ranks of the threatened.
Before the significant development of a global pet trade, several species were harvested for use as pickled specimens to dissect in high school or college anatomy classes. The northern leopard frog (Rana pipiens) in North America and the toads Bufo chilensis in Chile and Bufo arenarum in Argentina have all been collected by the thousands for educational and scientific purposes. These species are generally widespread and abundant (although the northern leopard frog has disappeared from parts of its former range in the midwestern United States) despite decades of persecution. This observation highlights the fact that while some species are threatened by over-harvesting, others can tolerate moderate levels of collecting without suffering serious population level declines.
Synergistic effects
Aside from habitat destruction, declines are rarely the result of single causes[50]. Disappearances of the jambato toad in Ecuador and Eleutherodactylus in Puerto Rico show a relationship with both unusual climate and the appearance of the chytrid fungal disease[51][52]. Mortality in boreal toads in the U.S. Pacific Northwest has resulted from of a combination of ultraviolet radiation and Saprolegnia fungus[53]. Even in the case of the mountain yellow-legged frog where introduced trout seemed to be a major explanation for declines, Bd may also play a role[54].
Other scenarios are also possible. Bullfrogs on a Uruguayan frog farm tested positive for chytrid disease in 1999. These frogs could have infected local native frogs, or brought the disease with them when they were exported to other South American countries or the United States[54]. The African clawed frog (Xenopus laevis) apparently tolerates Bd in its native Africa. Worldwide trade in the species in the mid-1900s as a pregnancy assay and later as a laboratory study animal may have spread the disease to susceptible native species[55]. The only known long-distance dispersal mechanism for the deadly chytrid disease involves movement on live frogs. Thus transport of frogs not only for meat but also for the pet trade has the potential to spread this disease to more parts of the world.
Notes This is a chapter from Disappearing Jewels: The Status of New World Amphibians (e-book). Previous: The Status of New World Amphibians (Disappearing Jewels: Chapter 3) |Table of Contents (Disappearing Jewels: Chapter 3)|Next: Conserving Amphibians: An Agenda for the Future
Citation
International, C., Nature, T., , N., Young, B., Stuart, S., Chanson, J., Cox, N., & Boucher, T. (2012). Disappearing Jewels: Chapter 3. Retrieved from http://editors.eol.org/eoearth/wiki/Disappearing_Jewels:_Chapter_3- ↑ Young, B. E., K. R. Lips, J. K. Reaser, R. Ibáñez, A. W. Salas, J. R. Cedeño, L. A. Coloma, S. Ron, E. La Marca, J. R. Meyer, A. Muñoz, F. Bolaños, G. Chaves, and D. Romo. 2001 Population declines and priorities for amphibian conservation in Latin America. Conservation Biology 15:1213-1223.
- ↑ Collins, J. P. and A. Storfer. 2003. Global amphibian declines: sorting the hypotheses. Diversity and Distributions 9:89-98.
- ↑ Population Division of the Department of Economic and Social Affairs of the United Nations Secretariat. 2001 and 2002. World Population Prospects: The 2002 Revision and World Urbanization Prospects: The 2001 Revision, downloaded 22 June 2004.
- ↑ Food and Agriculture Organization of the United Nations (FAO). 2001. Global Forest Resources Assessment 2000--main Report. FAO Forestry Paper No. 140. Food and Agriculture Organization, Rome, Italy.
- ↑ Dahl, T. E. 2000. Status and Trends of Wetlands in the Conterminous United States 1986 to 1997. United States Department of the Interior, Fish and Wildlife Service, Washington, D.C., USA.
- ↑ World Resources Institute. 2001. World Resources 2000-2001: People and Ecosystems: The Fraying Web of life. World Resources Institute, Washington, D.C., USA.
- ↑ World Commission on Dams. 2000. Dams and Development: A New Framework for Decision-Making. Earthscan Publications, London, UK.
- ↑ Lynch, J. D. and T. Grant. 1998. Dying frogs in western Colombia: catastrophe or trivial observation? Revista de la Academia Colombiana de Ciencias 22:149-152.
- ↑ Laurance, W. F., K. R. McDonald, and R. Speare. 1996. Epidemic disease and the catastrophic decline of Australian rain forest frogs. Conservation Biology 10:406-413.
- ↑ Lips, K. R. 1998. Decline of a tropical montane amphibian fauna. Conservation Biology 12:106-117.
- ↑ Pounds, J. A., and M. L. Crump. 1994. Amphibian declines and climate disturbance: the case of the golden toad and the harlequin frog. Conservation Biology 8:72-85.
- ↑ Scott, N. J. 1993. Postmetamorphic death syndrome. Froglog 7:1-2.
- ↑ Berger, L., R. Speare, P. Daszak, D. E. Green, A. A. Cunningham, C. L. Goggin, R. Slocombe, M. A. Ragan, A. D. Hyatt, K. R. McDonald, H. B. Hines, K. R. Lips, G. Marantelli, and H. Parkes. 1998. Chytridiomycosis causes amphibian mortality associated with population declines in the rainforests of Australia and Central America. Proceedings of the National Academy of Science (USA) 95:9031-9036.
- ↑ Longcore, J. E., A. P. Pessier, and D. K. Nichols. 1999. Batrachochytrium dendrobatidis gen. and sp. nov., a chytrid pathogenic to amphibians. Mycologia 91: 219-227.
- ↑ Emerging Wildlife Diseases: Threats to Amphibian Biodiversity.
- ↑ Barr, D. J. S. 1990. Phylum Chytridiomyceta. Pages 454-466 in L. Margulis, J. O. Corliss, M. Melkonia, and D. J. Chapman (editors), Handbook of Protoctista. Lubrecht and Kramer, Monticello, New York, USA.
- ↑ Powell, M. J. 1993. Looking at mycology with a Janus face: a glimpse of chytridiomycetes active in the environment. Mycologia 85:1-20.
- ↑ Bradley G. A., P. C. Rosen, M. J. Sredl, T. R. Jones, and J. E. Longcore J. E. 2002. Chytridiomycosis in native Arizona frogs. Journal of Wildlife Diseases 38:206-212.
- ↑ Morehouse, E. A., T. Y. James, A. R. D. Ganley, R. Vilgalys, L. Berger, P. J. Murphy, and J. E. Longcore. 2003. Multilocus sequence typing suggests the chytrid pathogen of amphibians is a recently emerged clone. Molecular Ecology 12:395-403.
- ↑ Burrowes, P. A., R. L. Joglar and D. E. Green. 2004. Potential causes for amphibian declines in Puerto Rico. Herpetologica 60:141-154.
- ↑ Carey, C., N. Cohen, and L. A. Rollins-Smith. 1999. Amphibian declines: an immunological perspective. Developmental and Comparative Immunology 23:459-472.
- ↑ Speare, R., Berger, L. 2000. Chytridiomycosis in amphibians in Australia. Web publication available at <a class="external free" href="http://www.jcu.edu.au/school/phtm/PHTM/frogs/chyspec.htm" rel="nofollow" title="http://www.jcu.edu.au/school/phtm/PHTM/frogs/chyspec.htm">http://www.jcu.edu.au/school/phtm/PHTM/frogs/chyspec.htm</a>.
- ↑ Johnson, M. and R. Speare. 2003. Survival of Batrachochytrium dendrobatidis in water: quarantine and control implications. Emerging Infectious Diseases 9:922-925.
- ↑ Morell, V. 1999. Are pathogens felling frogs? Science 284:728-731.
- ↑ Speare, R., Berger, L. 2000. Chytridiomycosis in amphibians in Australia. Web publication available at <a class="external free" href="http://www.jcu.edu.au/school/phtm/PHTM/frogs/chyspec.htm" rel="nofollow" title="http://www.jcu.edu.au/school/phtm/PHTM/frogs/chyspec.htm">http://www.jcu.edu.au/school/phtm/PHTM/frogs/chyspec.htm</a>.
- ↑ Lips, K. R., J. D. Reeve, and L. R. Witters. 2003. Ecological traits predicting amphibian population declines in Central America. Conservation Biology 17:1078-1088.
- ↑ Taylor, S. K., E. S.Williams and K. W. Mills. 1999. Effects of malathion on disease susceptibility in Woodhouse's Toads. Journal of Wildlife Diseases 35:536-541.
- ↑ Food and Agriculture Organization of the United Nations. 2003. FAOSTAT On-line Statistical Service. Food and Agriculture Organization, Rome.
- ↑ Hayes, T., A. Collins, M. Lee, M. Mendoza, N. Noriega, A. A. Stuart, and A. Vonk. 2002. Hermaphroditic, demasculinized frogs after exposure to the herbicide atrazine at low ecologically relevant doses. Proceedings of the National Academy of Sciences (USA) 99:5476-5480.
- ↑ Hayes, T., K. Haston, M. Tsui, A. Hoang, C. Haeffele, and A. Vonk. 2002. Feminization of male frogs in the wild. Nature 419:895-896.
- ↑ Davidson, C., H. B. Shaffer, and M. R. Jennings. 2002. Spatial tests of the pesticide drift, habitat destruction, UV-B, and climate-change hypotheses for California amphibian declines. Conservation Biology 16:1588-1601.
- ↑ Davidson, C., H. B. Shaffer, and M. R. Jennings. 2002. Spatial tests of the pesticide drift, habitat destruction, UV-B, and climate-change hypotheses for California amphibian declines. Conservation Biology 16:1588-1601.
- ↑ Lajmanovich, R. C., M. T. Sandoval, and P. M. Peltzer. 2003. Induction of mortality and malformation in Scinax nasicus tadpoles exposed to glyphosate formulations. Environmental Contamination and Toxicology 70:612-618.
- ↑ Izaguirre, M. F., R. C. Lajmanovich, P. M. Peltzer, A. P. Soler, and V. H. Casco. 2000. Cypermethrin-induced aptosis in the telencephalon of Physalaemus biligonigerus tadpoles (Anura: Leptodactylidae). Environmental Contamination and Toxicology 65:501-507.
- ↑ Intergovernmental Panel on Climate Change. 2001. Climate change 2001: The scientific basis. United Nations Environmental Program, New York.
- ↑ Parmesan, C. and G. Yohe. 2003. A globally coherent fingerprint of global change impacts across natural systems. Nature 421:37-42.
- ↑ Root, T. L., J. T. Price, K. R. Hall, S. H. Schneider, C. Rosenzweig, and J. A. Pounds. 2003. Fingerprints of global warming on wild animals and plants. Nature 421:57-60.
- ↑ Thomas, C. D., A. Cameron, R. E. Green, M. Bakkenes, L. J. Beaumont, Y. C. Collingham, B. F. N. Erasmus, M. F. de Sequeira, A. Grainger, L. Hannah, L. Hughes, B. Huntley, A. S. van Jaarsveld, G. F. Midgley, L. Miles, M. A. Ortega-Huerta, A. T. Peterson, O. L. Phillips, and S. E. Williams. 2004. Extinction risk from climate change. Nature 427:145-148.
- ↑ Thomas, C. D., A. Cameron, R. E. Green, M. Bakkenes, L. J. Beaumont, Y. C. Collingham, B. F. N. Erasmus, M. F. de Sequeira, A. Grainger, L. Hannah, L. Hughes, B. Huntley, A. S. van Jaarsveld, G. F. Midgley, L. Miles, M. A. Ortega-Huerta, A. T. Peterson, O. L. Phillips, and S. E. Williams. 2004. Extinction risk from climate change. Nature 427:145-148.
- ↑ BirdLife International. 2004. State of the World’s Birds 2004: Indicators for our Changing World. BirdLife International, Cambridge, UK.
- ↑ Still, C. J., P. N. Forster, and S. H. Schneider. 1999. Simulating the effects of climate change on tropical montane cloud forests. Nature 398:608-610.
- ↑ Lawton, R. O., U. S. Nair, R. A. Pielke Sr., and R. M. Welch. 2001. Climatic impact of tropical lowland deforestation on nearby montane cloud forests. Science 294:584-587.
- ↑ Pounds, J. A., M. P. L. Fogden, and J. H. Campbell. 1999. Biological response to climate change on a tropical mountain. Nature 398:611-615.
- ↑ Pounds, J. A. 2001. Climate and amphibian declines. Nature 410:639-640.
- ↑ Pounds, J. A., M. P. L. Fogden, and J. H. Campbell. 1999. Biological response to climate change on a tropical mountain. Nature 398:611-615.
- ↑ Ron, S. R., W. E. Duellman, L. A. Coloma, and M. R. Bustamante. 2003. Population decline of the Jambato Toad Atelopus ignescens (Anura: Bufonidae) in the Andes of Ecuador. Journal of Herpetology 37:116-126.
- ↑ Burrowes, P. A., R. L. Joglar and D. E. Green. 2004. Potential causes for amphibian declines in Puerto Rico. Herpetologica 60:141-154.
- ↑ Huacuz Elías, D. C. 2002. El Achoque del Lago de Pátzcuaro. Universidad Michoacana de San Nicolás de Hidalgo, Morelia, Mexico.
- ↑ Lescure, J. 1979. Étude taxonomique et eco-ethologique d’um amphibian des Petites Antilles: Leptodactylus fallax Müller, 1926 (Leptodactylididae). Bulletin du Muséum National d'Histoire Naturelle, Paris, 4e série, 1, sect. A (3):757-774.
- ↑ Corn, P. S. 2000. Amphibian declines: review of some current hypotheses. Pages 663-669 in D.W. Sparling, C. A. Bishop, and G. Linder (editors), Ecotoxocology of Amphibians and Reptiles. SETAC Press, Pensacola, Florida, USA.
- ↑ Burrowes, P. A., R. L. Joglar and D. E. Green. 2004. Potential causes for amphibian declines in Puerto Rico. Herpetologica 60:141-154.
- ↑ Ron, S. R., W. E. Duellman, L. A. Coloma, and M. R. Bustamante. 2003. Population decline of the Jambato Toad Atelopus ignescens (Anura: Bufonidae) in the Andes of Ecuador. Journal of Herpetology 37:116-126.
- ↑ Kiesecker, J. M., A. R. Blaustein, and L. K. Belden. 2001. Complex causes of amphibian declines. Nature 410:681-684.
- ↑ Mazzoni, R., A. A. Cunningham, P. Daszak, A. Apolo, E. Perdomo, and G. Speranza. 2003. Emerging pathogens of wild amphibians in frogs (Rana catesbeiana) farmed for international trade. Emerging Infectious Diseases 9:995-998.
- ↑ Weldon, C., L. H. du Preez, A. D. Hyatt, R. Muller, and R. Speare. 2004. Out of Africa: evidence for the origin of the amphibian chytrid fungus. Emerging Infectious Diseases, in press.


