Changes in aquatic biota and ecosystem structure and function in the Arctic
This is Section 8.4.4 of the Arctic Climate Impact Assessment
Lead Authors: Frederick J.Wrona,Terry D. Prowse, James D. Reist; Contributing Authors: Richard Beamish, John J. Gibson, John Hobbie, Erik Jeppesen, Jackie King, Guenter Koeck, Atte Korhola, Lucie Lévesque, Robie Macdonald, Michael Power,Vladimir Skvortsov,Warwick Vincent; Consulting Authors: Robert Clark, Brian Dempson, David Lean, Hannu Lehtonen, Sofia Perin, Richard Pienitz, Milla Rautio, John Smol, Ross Tallman, Alexander Zhulidov
Climate change is very likely to have both direct and indirect consequences on the biota and the structure and function of arctic freshwater ecosystems. Changes in key physical and chemical parameters described previously are very likely to affect community and ecosystem attributes such as species (Species richness) richness, biodiversity, range, and distribution, and consequently alter corresponding food web structures and primary and secondary production levels. The magnitude and extent of the ecological consequences of climate change in arctic freshwater ecosystems will depend largely on the rate and magnitude of change in three primary environmental drivers: the timing, magnitude, and duration of the runoff regime; temperature; and alterations in water chemistry such as nutrient levels, dissolved oxygen content (DOC), and particulate organic matter loadings[1].
Contents
Effects on biological communities, biodiversity, and adaptive responses (8.4.4.1)
Climate change will probably produce significant effects on the biodiversity of freshwater ecosystems throughout the Arctic and possibly initiate varying adaptive responses. The magnitude, extent, and duration of the impacts and responses will be system- and location-dependent, and difficult to separate from other environmental stressors. Biodiversity is related to, or affected by, factors including:
- the variability of regional and local climate;
- the availability of local resources (e.g., water, nutrients, trace elements, energy, substrate) affecting the productivity potential;
- the nature, timing, and duration of disturbance regimes in the area (e.g., floods, catastrophic water loss, fire);
- the original local and regional "stock" of species and their dispersal opportunities or barriers;
- the physiological capacity of individuals and populations to cope with new environmental conditions (e.g., physiological thresholds and tolerances);
- the levels of spatial heterogeneity (habitat fragmentation) and connections among aquatic systems;
- the intensity of biotic interactions such as competition, predation, disease, and parasitism;
- phenotypic and genotypic flexibility in reproductive and life-history strategies (e.g., facultative versus obligatory anadromy for certain fish; plasticity in sexual versus asexual reproductive strategies in aquatic invertebrate and plant species); and
- the overall genetic variability and adaptive capacity of the species[2].
Many arctic freshwater systems are exposed to multiple environmental stressors or perturbations including point- and/or nonpoint-source pollution (e.g., long-range aerial transport of contaminants; Section 8.7 (Changes in aquatic biota and ecosystem structure and function in the Arctic)); altered hydrologic regimes related to impoundments and diversions; water quality changes from landscape alterations (e.g., mining, oil and gas exploration); and biological resource exploitation (e.g., subsistence and commercial fisheries and harvesting of waterfowl and mammals; Section 8.5 (Changes in aquatic biota and ecosystem structure and function in the Arctic)), to name a few. These stressors, along with climate variability, can synergistically contribute to the degradation of biological diversity at the species, genetic, and/or habitat–ecosystem levels[3]. There is little evidence to suggest that climate change will slow species loss. There is growing evidence, however, that climate change will contribute to accelerated species losses at regional and global levels[4] and that the effects of alterations in the biodiversity of ecosystem structure and function are likely to be more dependent on given levels of functional diversity than on the total number of species[5]. Moreover, both the number and type of functional units present in a community largely affect ecosystem resilience and vulnerability to change[6].
For these reasons, large uncertainties remain in projecting species- and system-specific responses and the impacts of changes in climate and UV radiation levels on biodiversity at local and regional spatial scales. However, several broad projections can be made.
First, locally adapted arctic species are likely to be extirpated from certain areas as environmental conditions begin to exceed their physiological tolerances and/or ecological optima. Hence, species with limited climatic ranges and/or restricted habitat requirements (related to particular physiological or phenological traits) are very likely to be vulnerable to climate change effects. Species with low population numbers and/or that reside in restricted, patchy, and highly specialized environments will be particularly at risk[7]. While wholesale extinctions of entire arctic species are unlikely, some highly valued species (e.g., certain fish species) may possibly become geographically or ecologically marginalized. For example, there are pronounced north–south gradients in the taxonomic composition of stream macroinvertebrate communities in the Arctic, with decreasing species diversity and an increasing importance of taxa such as dipterans with distance northward[8]. Moreover, many of the high-latitude filamentous algal species have temperature optima well above the low ambient water temperatures at which they reside, and are therefore likely to respond positively to moderate increases in temperature[9]. Hence, many high-latitude species are currently at their physiological limits and are likely to be very sensitive to future shifts in climate[10]. Projected changes in regional runoff patterns and temperature regimes are very likely to affect river and stream environments, possibly reducing the severity of disturbance events that are an integral component of their current hydro-ecology (Section 8.4.2 (Changes in aquatic biota and ecosystem structure and function in the Arctic)). Specifically, Scrimgeour et al.[11] suggested that if these disturbances play a role in maintaining habitat complexity and associated species (Species richness) richness and diversity, then climate-related changes in the severity of these events will affect macroinvertebrate and aquatic algal species distribution and associated biodiversity patterns (see also Prowse and Culp[12]).
In estuarine [[habitat]s], there are likely to be shifts in species composition to more euryhaline and anadromous species (e.g., fourhorn sculpin – Myoxocephalus quadricornis, ninespine stickleback – Pungitius pungitius, threespine stickleback – Gasterosteus aculeatus, Arctic flounder – Pleuronectes glacialis, salmonines, and coregonines). Such shifts in species composition will possibly have cascading effects resulting from competition for food resources with marine species (e.g., Arctic cod – Boreogadus saida) that currently inhabit many estuarine zones. The subsequent effects on higher trophic levels (e.g., the impact of potentially decreased Arctic cod abundance on marine mammals and birds) remains unknown (see also Section 9.3.4 (Changes in aquatic biota and ecosystem structure and function in the Arctic)).
For other fish species (e.g., Arctic char), alterations in environmental conditions could possibly shift or reduce the availability of preferred habitats of certain morphs, leading, in the extreme case, to the extirpation of particular morphs from certain locations. For example, pelagic forms of Arctic char in Thingvallavatn, Iceland, occupy portions of the water column that experience summer heating. Should such heating ultimately exceed thermal preferences for this morph, its growth is likely to decrease, with a concomitant reduction in reproduction and productivity. Ultimately, exclusion from the habitat during critical times could possibly occur, permanently extirpating that morph from such areas.
Changes in habitat characteristics driven by climate change are also likely to differentially affect specific populations of fish. For example, some aspects of life-history variation in Dolly Varden on the Yukon north slope appear to be particularly associated with inter-river variation in groundwater thermal properties (e.g., egg size is larger and development time is shorter in [[river]s] that have significant groundwater warming, and reproduction occurs annually in these warmer rivers because sea access allows for earlier feeding, compared to reproduction every two years or less often in colder rivers[13]). Thus, climate change effects that mimic this natural local inter-population variability are likely to result in similar shifts in populations presently occupying colder habitats.
A second major effect of climate change will probably be alterations in the geographic range of species, thereby affecting local and regional biodiversity. This is likely to occur through a combination of compression or loss of optimal habitat for "native" arctic species, and the northward expansion of "non-native" southern species. For instance, the large number of northward-flowing arctic rivers provides pathways for colonization of the mainland by freshwater species that, due to climatic limitations, are presently restricted to subarctic or temperate portions of the drainage basins. As climate change effects become more pronounced (e.g., degree-day boundaries or mean temperature isotherms shift northward), the more ecologically vagile species are likely to extend their geographic ranges northward[14]. In North America, for example, the distribution of yellow perch (Perca flavescens) is projected to expand northward beyond its current, primarily subarctic distribution. Traditional ecological knowledge from the western Canadian Arctic has identified new species of fish (Pacific salmon – Oncorhynchus spp. and least cisco – Coregonus sardinella) that were not previously present in some aquatic systems of the area[15] (see also Chapter 3 (Changes in aquatic biota and ecosystem structure and function in the Arctic), specifically Fig. 3.2). The complete consequences of such new colonizations are unknown, but could include the introduction of new diseases and/or parasites; population reduction or extirpation through competition for critical resources; increased predation; increased hybridization of closely related taxa; and others (see sections 8.5.1.1 (Changes in aquatic biota and ecosystem structure and function in the Arctic) and 8.5.2 (Changes in aquatic biota and ecosystem structure and function in the Arctic) for detailed discussions of climate-related range extensions in selected fish species and their potential ecological consequences).
Emergent aquatic plants are also expected to expand their distribution northward and thus alter the overall levels of primary production in ponds and small lakes in the Arctic. Alexander et al.[16] reported total primary production of 300 to 400 g C/m2/yr in ponds of emergent Carex (covering one-third of the pond) in Barrow, Alaska, compared to total primary production of 1 g C/m2/yr for phytoplankton and 10 g C/m2/yr for epilithic algae. Traditional ecological observations by trappers on the Peace-Athabasca Delta of the Mackenzie River system, Canada, suggest that muskrat abundance is likely to increase in high-latitude lakes, ponds, and wetlands as emergent aquatic vegetation becomes more prominent[17]. While the potential northern limit for emergent aquatic macrophytes is not fully known, their projected increased presence will clearly influence the overall productivity and structural complexity of arctic pond and lake [[habitat]s].
An overarching issue affecting the responses of arctic aquatic biota and related biodiversity to rapid climate change is "adaptive capacity". The magnitude of change in arctic climate projected for the next 100 years does not exceed that experienced previously, at least at a geological timescale. The future rate of change however, is very likely to be unprecedented. To survive such a challenge, arctic aquatic biota, especially those that are truly arctic in nature, must have the inherent capacity to adapt (i.e., have sufficient genetic capacity at the population level to evolve at the required rate); acclimate (i.e., the phenotypic ability at the population and/or individual level to survive in the new conditions); and/or move (i.e., emigrate to more optimal situations). High levels of diversity that are present below the species level in many arctic organisms imply that some evolutionary compensation for rapid climate change is possible. Taxa with short generation times (e.g., zooplankton) will be able to evolve more rapidly than those with longer generation times (e.g., fish). Furthermore, assessment of genetic variability for some taxa (e.g., mitochondrial DNA in Arctic char[18]) suggests that previous events that reduced genetic diversity may have limited their capacity for such rapid evolution. This will probably further hamper responses by such taxa and, with the projected rapid rate of climate change and other factors (e.g., competition from new colonizers), is likely to result in an increased risk of local extirpation and/or extinction.
Many arctic taxa may already be pre-adapted to acclimate successfully to rapid change. For example, many organisms already have enzymes with different thermal optima to allow them to cope with changing environmental conditions. Such capacity, which is presumed but not demonstrated to exist in most arctic taxa, could possibly counterbalance the increased risk of extinction noted above. Taxa that are capable of emigrating to new areas have additional options to cope with rapid climate change, although access issues are likely to preclude such movements to suitable conditions.
Clearly, significant changes in aquatic biodiversity are very likely to result from climate change, and biota have varying capacities to cope with the rate of this change. Ecologically speaking, any change will have significant ramifications in that adjustments in the ecosystem will follow (sections 8.4.4.2, 8.4.4.3, 8.4.4.4, and 8.5]]). However, from the human perspective, important questions surround the perceived significance of such changes from economic, cultural, and value perspectives (see Chapters 3, 11, and 12 (Changes in aquatic biota and ecosystem structure and function in the Arctic) for discussions of possible socioeconomic implications).
Effects on food web structure and dynamics (8.4.4.2)
The impacts of climate change on the structure and dynamics of aquatic [[food web]s] remain poorly understood. To date, many of the insights as to how arctic food webs will respond (directly or indirectly) to climate change effects have been obtained from either descriptive studies or a select few manipulative/experimental studies where ecosystem-level or food web manipulations were conducted and response variables measured. Stream processes and biotic populations of the Kuparuk River and Oksrukuyik Creek, Alaska, have been shown to be controlled by the geomorphology of the systems (i.e., input from nutrient-rich springs[19]); climate (i.e., precipitation affects discharge, which affects insect and fish production[20]); resource fluxes from the surrounding catchments[21]; and corresponding biotic interactions. For example, nutrient enrichment of the streams resulted in greater primary and fish production, and a corresponding increase in the abundance of benthic macroinvertebrates[22]. In addition, after seven years of artificial enrichment of the Kuparuk River, the dominant primary producers changed from diatoms to mosses[23], which subsequently altered the abundance, distribution, and taxonomic composition of the macroinvertebrate community[24].
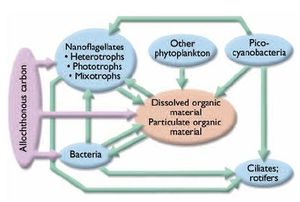
Other recent studies of arctic systems have identified the structural and functional importance of the microbial freshwater food web (Fig. 8.13). Work in this area has shown that the microbial food web can comprise a significant fraction of the total community biomass in arctic [[river]s] and lakes, and that energy flow is routed through a diverse trophic network of microbial species displaying a wide array of nutritional modes (heterotrophic bacteria, phototrophic bacteria, phagotrophic protozoa, and mixotrophic flagellates[25]). How climate change will influence the response of the microbial food web is not entirely certain, but studies of temperate systems might help provide insight. Interestingly, research on microbial food webs of more temperate aquatic systems shows that in the absence of heavy grazing pressure on bacteria by macrozooplankton or benthic macroinvertebrates, the principal role of the microbial food web is the degradation (respiration) of organic matter[26]. Hence, the microbial food web is a significant source of energy to plankton, being largely responsible for recycling nutrients in the water column and thereby helping to sustain planktonic and benthic primary production and ultimately higher secondary and tertiary consumers in the food chain[27]. Projected increases in water temperature and inputs of DOC, particulate organic carbon (POC), and DIC arising from climate change are very likely to affect the structural and functional dynamics of the microbial food web, and are likely to increase rates of carbon processing. Pienitz et al.[28] showed that the same abiotic parameters, along with lake morphometry, explain the greatest percentage of variance in diatom community composition in northwestern Canada. Furthermore, diatom community structure was highly correlated with DOC gradients in Siberian and subarctic Québec lakes[29]. Hence, concomitant changes in the phytoplankton component of the food web probably will also cascade through the ecosystem.
Increasing temperature has the potential to alter the physiological rates (e.g., growth, respiration) of individuals, and the vital rates and resulting dynamics of populations[30]. Mesocosm studies by Beisner et al.[31], which investigated the influence of increasing temperature and food chain length on plankton predator–prey dynamics, showed that the predator–prey system is destabilized at higher temperatures (i.e., the macrozooplankton herbivore Daphnia pulex always became extinct), irrespective of the complexity of the food web (i.e., whether a two- or three-level food web was involved). Long-term studies of Toolik Lake, Alaska, project that rising temperatures are likely to eliminate lake trout (Salvelinus namaycush) populations in this lake, with concomitant impacts on the food web. The bioenergetics model used by McDonald M.E. et al.[32] projects that a 3°C rise in July epilimnetic (surface mixed-layer) temperatures could cause young-of-the-year lake trout to require eight times more food than at present just to maintain adequate condition. This requirement greatly exceeds the current food availability in the lake, although it is probable that food availability will increase as temperatures rise. Furthermore, the oxygen concentrations projected by the lake model[33] show that a future combination of higher temperatures and increased loading of total phosphorus would greatly reduce the hypolimnetic (bottom:water) habitat available for lake trout.
An example of top-down control through size-selective predation was found in ponds and lakes in Barrow, Alaska: lakes with fish had small and transparent Daphnia longiremis, while lakes without fish and all ponds had large and pigmented D. middendorffiana and D. pulex as well as fairy shrimp and the copepod Heterocope spp.[34]. Rouse et al.[35] concluded that since top predators (fish) in arctic systems tend to be long-lived, population changes owing to recruitment failure may not be reflected in the adult populations for many years. However, the effects of the eventual loss of top predators from these systems are likely to cascade through the food web, affecting the structure and function of both benthic and planktonic communities[36].
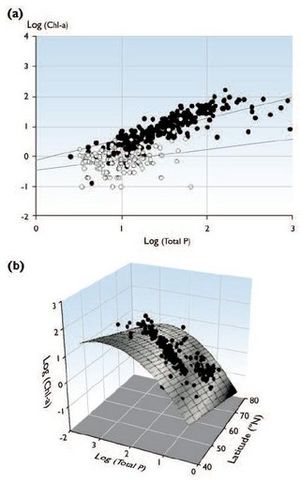
Given the information presented in this section, it is very probable that climate change will substantially affect biological interactions, including trophic structure and food chain composition. With top-down and bottom:up processes operating simultaneously in ecosystems[38], the degree to which each process influences producer biomass varies[39]. Consequently, the well-established relationship between phosphorus and algal biomass may differ between systems with different levels of productivity. For example, in a two-level trophic system (relatively unproductive), grazing zooplankton may control the algal biomass and the expected positive relationship between chlorophyll-a (Chl-a) and total phosphorus (P) would not be observed. Therefore, differences in productivity and trophic level interactions may explain the discrepancy in the Chl-a–total P relationship between temperate and arctic lakes (Fig. 8.14). The low productivity that has been observed in many arctic lakes may limit the presence of fish predators (i.e., more closely represent a two-level trophic system) and may result in systems where algal biomass is controlled by extensive zooplankton grazing[40].
Top-down control of food web structure in arctic stream and river ecosystems is also important. Golden and Deegan[41] found that young Arctic grayling (Thymallus arcticus) have the potential to produce top-down cascading trophic effects in arctic streams where nutrients are not limited. The grayling were found to affect trophic structure through consumption, nutrient excretion, and the modification of prey behavior. Epilithic Chl-a increased with increasing fish density in both reference (P-limited) and fertilized (P-enriched) zones of the Kuparuk River, Alaska, while mayfly density decreased with increasing fish density in the fertilized zone only. These results further illustrate that projecting climate change impacts is not straightforward.
Effects on primary and secondary production (8.4.4.3)
Primary and secondary productivity relationships in arctic aquatic ecosystems are highly susceptible to structural and functional alterations resulting from changes in climate, although the direction and absolute magnitude of the responses are likely to be difficult to project[42]. For example, while constituents of microbial [[food web]s] (e.g., the picocyanobacteria, heterotrophic bacteria, etc.; Fig. 8.13) are likely to respond positively to temperature increases, the photosynthesis rate in the picoplankton fraction (0.2–2 µm) is strongly stimulated by increased temperature to a greater extent than nanoplankton (2–20 µm) and microplankton (20–200 µm) fractions[43].
In general, lake primary productivity will probably increase because higher temperatures correlate with higher primary productivity (a longer ice-free season and more sunlight before the summer solstice are very likely to result in greater primary production by plankton; see Box 8.5). Brylinsky and Mann[44] analyzed lake productivity in 55 lakes and reservoirs from the tropics to the Arctic, and found the best abiotic variables for estimating productivity to be latitude and air temperature. A closer examination of the relationship between total P, total nitrogen, latitude, and algal biomass (n=433 lake years) also revealed that average algal biomass during the ice-free season is significantly negatively related to the latitude of the system, independent of the nutrient concentration[45]. This strong latitudinal effect on algal biomass yield suggests that arctic lakes are likely to show a significant increase in productivity if temperature and nutrient loadings in these systems increase as scenarios of future climate change project. While Shortreed and Stockner[46] found that arctic lakes have lower primary productivity than temperate lakes, Flanagan et al.[47] showed that at a given level of phosphorus, the productivity of arctic lakes is significantly less than lakes in the temperate zone, with the biomass of lower (trophic level) producers not accounted for simply by lower nutrient concentrations in the Arctic. Further examination of detailed observations of phytoplankton community structure from arctic Long-Term Ecological Research sites indicates that there is no fundamental shift in taxonomic group composition between temperate and arctic phytoplankton communities. This suggests that the difference in the Chl-a–total P relationship between temperate[48] and arctic lakes is not an artifact of changes in the Chl-a–biomass ratio resulting from a taxonomic shift in algal communities[49]. Hence, the observed difference in the Chl-a–total P relationship for temperate and arctic lakes may provide insight about the future effects of climate change (Fig. 8.14).
|
Box 8.5. Productivity of northeastern Greenland lakes: species composition and abundance with rising temperatures The Danish BioBasis monitoring program (part of Zackenberg Ecological Research Operations), initiated in 1995, includes two lakes located in the Zackenberg Valley, northeastern Greenland (74º N).The monitoring at Zackenberg is expected to continue for at least 50 years.The area is situated in a high-arctic permafrost area in North-East Greenland National Park. More information about the area and the monitoring program can be found in Meltofte and Thing[50] and Christoffersen and Jeppesen[51], as well as at http://biobasis.dmu.dk. The two lakes, Sommerfuglesø and Langemandssø, have areas of 1.7 and 1.1 ha and maximum depths of 1.8 m and 6.1 m, respectively. Lakes and ponds in the area are ice-covered for most of the year, except from the end of July to the beginning of September. Most water bodies probably freeze solid during the late winter and spring. Primary producers in these nutrient-poor lakes are dinophytes, chlorophytes, and diatoms. Zooplankton grazers are sparse, consisting of Daphnia, copepods, and protozoans. Benthic invertebrates include Lepidurus.There are no fish in Sommerfuglesø. Dwarf Arctic char in Langemandssø prey on Daphnia, therefore, copepods and rotifers dominate zooplankton populations in this lake. Plants and animals in Sommerfuglesø and Langemandssø are active prior to ice melt, thus phytoplankton biomass becomes substantial as incoming solar radiation increases[52]. However, primary productivity slows as nutrients are consumed, and phytoplankton density varies annually with nutrient abundance. For example, in warmer years, nutrient concentrations and thus productivity are higher due to increased loading of nutrients and humus from catchments as the active layer thaws. Monitoring of plankton species in the two lakes during 1999 (a year of late ice melt and low water temperatures) and 2001 (a year of early ice melt and high water temperatures) has shown that not only do biomass and abundance change with temperature, but species composition changes as well. In 1999, when water temperatures were lower, chrysophytes and dinophytes represented 93% of total phytoplankton abundance in Sommerfuglesø, while dinophytes dominated phytoplankton (89% of total abundance) in the deeper and colder Langemandssø. In 2001, when ice broke early and water temperatures were higher, chrysophytes completely dominated phytoplankton in both lakes (94–95% of total phytoplankton abundance). Compared to 1999, total phytoplankton abundance was approximately twice as great in 2001, when nutrient levels were higher as well. Zooplankton abundance, in turn, was 2.5 times greater in 2001 than in 1999 in both lakes, likely in response to greater phytoplankton abundance. Daphnia and copepods were more abundant in 2001, while rotifers were less abundant than in 1999, perhaps in response to competition for food resources. 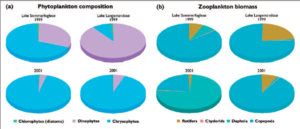 Fig. 1. (a) Phytoplankton composition (average of three samples per year) in Langemandssø and Sommerfuglesø in 1999 and 2001. In both years, total plankton abundance in Langemandssø was twice that in Sommerfuglesø; (b) relative biomass (dry weight) of different zooplankton groups in mid-August 1999 and 2001 in Langemandssø and Sommerfuglesø. In both years, total zooplankton biomass in Sommerfuglesø was approximately twice that in Langemandssø[53]. The climate of northeastern Greenland is projected to become more maritime in the future. Based on the five years of monitoring at Zackenberg thus far, increasing temperature and precipitation are projected to have major impacts on physicochemical and biological variables in the lakes. If snowfall increases, ice-cover duration is likely to be prolonged, shortening the growing season and reducing productivity, and possibly reducing food availability to the top predator in arctic lakes, the Arctic char. Greater runoff will probably increase nutrient loading and primary productivity, which could possibly result in oxygen depletion and winter fish kill.Thus, one probable outcome of climate change will be the extirpation of local fish populations in shallow lakes in similar ecological situations. Increased particulate loading is likely to limit light penetration for photosynthesis. Increased humus input with snowmelt is also likely to limit light penetration, reducing UV radiation damage to biota. |
Primary productivity is likely to increase if climate conditions at high latitudes become more suitable for industrial development, and if the associated pollution of currently nutrient-poor aquatic systems increases. For example, mountain lakes in the Kola Peninsula (e.g., Imandra Lake) and lakes and ponds in the Bolshezemelskaya tundra are currently stressed by heavy loadings of anthropogenic organic matter, heavy metals, and crude oil and drilling fluid, as well as thermal pollution. Phytoplankton structure (e.g., species) in these systems has changed, and primary as well as secondary productivity and biomass have increased significantly.
Arctic lakes, although relatively unproductive at present, will probably experience a significant increase in productivity as climate changes. If temperature and nutrient loads increase as projected, it is likely that phytoplankton will no longer experience temperature-induced photosynthetic rate inhibition, and growth rates will probably become more similar to those in the temperate zone, thus allowing for a greater accumulation of algae. If algae are heavily grazed by herbivores (Herbivory) at present because of a lack of predation, higher-level predators are likely to invade as the productivity of the system increases. Subsequent increased predation of the grazer community would permit an increase in algal biomass. In addition, the projected increase in nutrient concentration would augment these changes, making the increase in productivity even more dramatic.
Several empirical studies support this hypothesis. One study compared Swedish lakes (three-level trophic system) to unproductive antarctic lakes (two-level trophic system). The slope of the Chl-a–total P relationship for the antarctic lakes was significantly less than the Swedish lakes. This was hypothesized to be a consequence of the different trophic structures of the lakes, since productive (three-level) Swedish lakes showed a Chl-a–total P relationship similar to temperate lakes, suggesting that climate-related abiotic factors were not causing the differences between the Swedish and unproductive antarctic lakes[54]. Other empirical evidence supporting this hypothesis comes from subarctic lakes in the Yukon, which showed higher levels of zooplankton biomass relative to P concentrations than in temperate regions, suggesting a two-level trophic system. Shortreed and Stockner[55] attributed the high abundance of zooplankton to the low abundance of planktivorous fish, which led to an over-consumption of algae.
However, a significant factor further complicating the possible productivity response in arctic systems is the interaction of productivity with DOC. High DOC levels can differentially affect measured primary productivity by influencing light penetration (more DOC leads to darker water), affecting turbidity, and adding carbon for processing. For example, benthic diatoms and total diatom concentrations increased significantly during conditions of high DOC concentrations and low water transparency, whereas planktonic forms decreased[56]. In Southern Indian Lake, northern Manitoba, Hecky and Guildford[57] found that high DOC concentrations decreased light penetration sufficiently to cause a switch from nutrient to light limitation of primary production. In shallow tundra ponds, over 90% of algal primary production was by benthic algae[58], although this level of productivity is very likely to decline if there is appreciable DOC-related light reduction. By contrast, increased DOC is likely to reduce harmful UV-B radiation levels, and thereby have a possible countervailing effect on productivity[59].
Changes in primary productivity resulting from climate change, whether attributed to increased water [[temperature]s] or increased DOC loading, are likely to affect secondary production in arctic [[freshwater]s]. Productivity of lake zooplankton is very likely to rise in response to increases in primary production. At Toolik Lake, a 12-fold increase in primary production yielded a less than 2-fold increase in secondary production[60]. This enhanced production is very likely to result in an increase in the abundance of secondary producers, as observed in Alaska, where the abundance of microplankton (rotifers, protozoans) rose with increased primary production[61]. Although larger zooplankton showed little species change with increasing productivity, microzooplankton increased both in number of species (Species richness) (i.e., biodiversity) and trophic levels (i.e., productivity). Observations by Kling et al.[62] indicated that zooplankton abundance and diversity are more sensitive to changes in primary productivity with latitude, with species number and types declining to the north, than to changes in lake primary productivity at any given latitude. Therefore, lake productivity and species abundance and diversity will probably shift in favor of zooplankton as primary production increases in a progressively northward direction with climate change.
Climate change is unlikely to affect [[bacteria]l] species assemblages. Bahr et al.[63] found that the species of plankton in Toolik Lake, Alaska, were identical to species found in other lakes in temperate regions. The overall productivity of the lake did not appear to be related to the species of bacteria involved, instead, the total bacterial biomass in the plankton was affected by overall primary production and by the amount of allochthonous organic matter entering the lake from the drainage basin (Watershed). Crump et al.[64] and O’Brien et al.[65] reported that over half of the bacterial productivity in Toolik Lake was based on terrestrial DOC. As a result, the bacterial numbers, biomass, and productivity in this lake are many times higher than they would be if it contained a plankton-based microbial food web. In contrast, protozoa and rotifer communities are likely to change with the increasing primary productivity that will probably result from climate change. For example, major changes occurred in protozoan, algal, and rotifer assemblages and production owing to significant artificial nutrient additions (four times ambient loading rates[66]).
Effects on carbon dynamics (8.4.4.4)

The [[Arctic Climate Impact Assessment] (ACIA) (Arctic Climate Impact Assessment (full report))]-designated models project that by 2080, the majority of the Arctic will experience increases in air temperature (Section 4.4.2 (Changes in aquatic biota and ecosystem structure and function in the Arctic)), precipitation (Section 4.4.3 (Changes in aquatic biota and ecosystem structure and function in the Arctic)), evaporation (Section 6.2.3 (Changes in aquatic biota and ecosystem structure and function in the Arctic)), available degree-days for biological growth, and major changes in the extent and nature of permafrost (Section 6.6.1.3 (Changes in aquatic biota and ecosystem structure and function in the Arctic)). Although there are variations between model projections, permafrost degradation is projected to occur most extensively at more southerly latitudes of the circumpolar Arctic, with regional west-to-east gradients across North America and Eurasia (for distribution among ACIA regions see Figs. 6.23 and 8.2). Overall (based on a "median" model projection; [[Section 6.6.1.3 (Changes in aquatic biota and ecosystem structure and function in the Arctic)]2]), total permafrost area is projected to decrease by 11, 18, and 23% by 2030, 2050, and 2080, respectively. The loss of permafrost and deepening of the active layer is projected to be greatest in the western and southern areas of arctic and subarctic North America and Eurasia because initial permafrost temperatures are closer to 0°C than in more easterly and northern areas, and these areas are more likely to become snow-free earlier in the spring, permitting enhanced soil warming[67] (Section 6.4.4 (Changes in aquatic biota and ecosystem structure and function in the Arctic)). Growing degree-days are projected to increase across the Arctic (Fig. 8.15), with the greatest increase in Regions 1 and 4, with the exception of Greenland where growing degree-days are projected to remain the same or decrease. On average, a 20- to 30-day increase in growing-season length is projected for areas north of 60° N by the end of the 21st century. As the number of degree-days increases in the Arctic, carbon cycling in arctic wetlands is very likely to not only be affected by changes in the rates and magnitudes of primary and microbial productivity, but also in the quantity and quality of organic material that accumulates in these systems. This in turn will affect carbon loading to, and processing within, arctic lakes and [[river]s].
Wetlands are a very prominent feature of the Arctic, and are particularly sensitive to climate change. The structure of these systems, and their function as net sources or sinks of carbon, is likely to respond dramatically to changes in permafrost thawing, peatland distribution, and air temperatures and water budgets.
Thawing of perennially frozen wetland soil and ice is likely to result initially in a substantial efflux of carbon, as perennial stores of CO2 and CH4 are released to the atmosphere. Such an effect accounted for an estimated 1.6- to 3-fold increase in carbon emissions from degrading permafrost along the 0°C isotherm in Canada[68]. Permafrost thaw and warming has also accounted for a 100-fold increase in the rate of CO2 and CH4 formation in the Ob River basin[69], and is responsible for drastically increased effluxes of these two gases from a high-latitude mire in Sweden[70]. This initial increase in CO2 and CH4 emissions with permafrost thaw has potential positive climate feedbacks. The effect is likely to decline over time as gas stores are depleted, and as wetland vegetation, hydrology, and carbon sink/source function progressively change with climate.
Permafrost thaw and a greater number of growing degree-days are very likely to result in increased distribution and biomass of wetland vegetation at more northerly latitudes, increasing carbon storage in arctic and subarctic landscapes. Projections based on doubled atmospheric CO2 concentrations[71] indicate a probable 200 to 300 km northward migration of the southern boundary of peatlands in western Canada, and a significant change in their structure and vegetation all the way to the coast of the Arctic Ocean. Increases in carbon accumulation have been associated with peatland expansion, along with northward movement of the treeline, during Holocene warming, a process that slowed and eventually reversed with the onset of the Little Ice Age[72] (Section 8.3.2 (Changes in aquatic biota and ecosystem structure and function in the Arctic)). Similar expansion of peatlands and enhanced biomass accumulation have been recorded in North America in more recent times[73]. Hence, as [[temperature]s] rise, wetland/ peatland distribution is likely to increase at high latitudes, and arctic landscapes are likely to become greater carbon sinks. Carbon accumulation at high latitudes is likely to be limited by loss due to disturbance (e.g., increased occurrence of fire as temperatures and evapotranspiration increase in some areas[74]), which will possibly result in greater carbon loading to lakes and [[river]s][75].
Changes in available growing degree-days, along with changes in the energy and water balances of high-latitude wetlands, will have varying effects on the rates and magnitudes of photosynthetic assimilation of CO2, and anaerobic and aerobic production of CO2 and CH4 in existing arctic and subarctic wetlands:
- Rates and magnitudes of primary productivity, and hence carbon sequestration, are very likely to increase in arctic wetlands as air and soil temperatures rise, growing season lengthens (e.g., Greenland[76], Finland[77]), and as vegetation changes (as discussed above in the context of permafrost degradation). Carbon fixation in arctic and subarctic wetlands will, however, possibly be limited by UV radiation effects on vegetation[78] (see also Sections 7.4.2 and 8.6 (Changes in aquatic biota and ecosystem structure and function in the Arctic)).
- Carbon dioxide accumulation in high-latitude wetlands is likely to be limited by warming and drying of wetland [[soil]s], and the associated production and loss of CO2 through decomposition (e.g., Alaska: three-fold increase[79], Finland[80]). This effect is likely to lead to substantial losses of CO2 and potential climate feedbacks.
- Methane production and emissions are likely to decline as high-latitude wetland soils dry with rising [[temperature]s] and increased evapotranspiration, and with regional declines in precipitation (e.g., Finland[81], Greenland[82]). Moore T. and Roulet[83] have suggested that only a 10 cm deepening of the water table in northern forested peatlands results in their conversion from a source to a sink of atmospheric CH4. Methanotrophy is likely to be most pronounced in drier wetlands that tend toward aerobic conditions. The projected shift in vegetation toward woody species will possibly also limit CH4 release to the atmosphere[84] (Section 7.5.3.2 (Changes in aquatic biota and ecosystem structure and function in the Arctic)).
- Methane production in some wetlands is likely to increase as temperatures and rates of methanogenesis increase, and as water tables rise in response to regional increases in water availability (e.g., Finland – projected 84% increase in CH4 release from wet fen with 4.4°C temperature increase[85], Alaska – 8 to 33-fold increase in CH4 emissions with high water table[86]). Methane production will probably increase in those wetlands that have highly saturated soils and standing water, and those that may become wetter with future climate change, with potential climate feedbacks.
Overall, arctic and subarctic wetlands are likely to become greater sources of CO2 (and in some instances CH4) initially, as permafrost melts, and over the long term, as wetland soils dry[87]. Although many high-latitude wetlands are likely to experience a net loss of carbon to the atmosphere under future climate change, the expansion of wetland (e.g., peatland) distribution in the Arctic, and the increase in carbon accumulation with permafrost degradation, is likely to offset this loss (see Section 7.5 (Changes in aquatic biota and ecosystem structure and function in the Arctic) for further treatment of this topic).
In addition to wetlands, wholly aquatic systems ([[river]s], lakes, and ponds) are also important to carbon cycling in the Arctic. Kling et al.[88] showed that high-latitude lakes in Alaska were net producers of DOC, whereas [[stream]s] were typically net consumers. Many arctic lakes and rivers are supersaturated with CO2 and CH4, often emitting these gases to the atmosphere via diffusion; increases in productivity (e.g., primary and secondary) deplete carbon in surface waters, resulting in diffusion of CO2 into the water[89] (see Fig. 8.7). Kling et al.[90] found that coastal freshwater systems release carbon in amounts equivalent to between 20 and 50% of the net rates of carbon accumulation in tundra environments. Enhanced loadings of carbon to arctic lakes and rivers as permafrost degrades (surface and groundwater flows contribute dissolved CO2 and CH4, as well as POC) will affect carbon cycling in these systems in a number of ways.
Dissolved organic carbon loading of lakes and rivers is likely to result in increased primary productivity and associated carbon fixation. This increase in photosynthetic CO2 consumption by aquatic vegetation (e.g., algae, macrophytes) will possibly reduce emissions of this gas from lake waters to the atmosphere. This effect has been noted in experimental fertilization of both temperate and arctic lakes[91]. Nutrient loading of high-latitude rivers, however, is unlikely to have a similar effect, as these waters have a rapid rate of renewal.
Although DOC loading of surface waters will possibly cause a decline in CO2 emissions from some lakes, increased inputs of DOC and POC will possibly offset this effect and, in some cases, increase CO2 production. Enhanced DOC and POC loads increase turbidity in some lakes, reducing photosynthesis (See Effects of primary and secondary production above]]). This rise in the availability of organic matter will probably result in a concomitant increase in benthic microbial respiration, which produces CO2[92]. These effects are likely to be less pronounced or absent in flowing-water systems.
Increased nutrient loading and water temperature in high-latitude freshwater bodies are also likely to enhance methanogenesis in sediments. Slumping of ice-rich Pleistocene [[soil]s] has been identified as a major source of CH4 release from thermokarst lakes, such as in extensive areas of north Siberian lakes[93], and may explain high winter concentrations of atmospheric CH4 between 65° and 70° N[94]. Methane produced in such systems is often released to the atmosphere via ebullition, a process that will probably increase as the open-water season lengthens. Emission of CH4 to the atmosphere is also likely to be enhanced in lakes, ponds, and [[stream]s] that experience an increase in macrophytic growth, and an associated increase in vascular CH4 transport.
Chapter 8: Freshwater Ecosystems and Fisheries
8.1. Introduction (Changes in aquatic biota and ecosystem structure and function in the Arctic)
8.2. Freshwater ecosystems in the Arctic
8.3. Historical changes in freshwater ecosystems
8.4. Climate change effects
8.4.1. Broad-scale effects on freshwater systems
8.4.2. Effects on hydro-ecology of contributing basins
8.4.3. Effects on general hydro-ecology
8.4.4. Changes in aquatic biota and ecosystem structure and function
8.5. Climate change effects on arctic fish, fisheries, and aquatic wildlife
8.5.1. Information required to project responses of arctic fish
8.5.2. Approaches to projecting climate change effects on arctic fish populations
8.5.3. Climate change effects on arctic freshwater fish populations
8.5.4. Effects of climate change on arctic anadromous fish
8.5.5. Impacts on arctic freshwater and anadromous fisheries
8.5.6. Impacts on aquatic birds and mammals
8.6. Ultraviolet radiation effects on freshwater ecosystems
8.7. Global change and contaminants
8.8. Key findings, science gaps, and recommendations
References
- ^ Vincent, W.F. and J.E. Hobbie, 2000. Ecology of Arctic lakes and rivers. In: M. Nuttall and T.V. Callaghan (eds.). The Arctic: Environment, People, Policy, pp. 197–231. Harwood Academic Press.
Citation
Committee, I. (2012). Changes in aquatic biota and ecosystem structure and function in the Arctic. Retrieved from http://editors.eol.org/eoearth/wiki/Changes_in_aquatic_biota_and_ecosystem_structure_and_function_in_the_Arctic- ↑ Poff, N.L., M.M. Brinson and J.W. Day, 2002. Aquatic Ecosystems and Global Climate Change. Pew Center on Global Climate Change, Arlington, Virginia, 45pp.–Rouse, W.R., M.S.V. Douglas, R.E. Hecky, A.E. Hershey, G.W. Kling, L. Lesack, P. Marsh, M. McDonald, B.J. Nicholson, N.T. Roulet and J.P. Smol, 1997. Effects of climate change on the freshwaters of Arctic and subarctic North America. Hydrological Processes, 11:873–902.–Vincent, W.F. and J.E. Hobbie, 2000. Ecology of Arctic lakes and rivers. In: M. Nuttall and T.V. Callaghan (eds.).The Arctic: Environment, People, Policy, pp. 197–231. Harwood Academic Press.
- ↑ IPCC, 2001a. Climate Change 2001: Synthesis Report. A Contribution of Working Groups I, II and III to the Third Assessment Report of the Intergovernmental Panel on Climate Change. R.T. Watson and Core Writing Team. Cambridge University Press, 398pp.–Pimm, S.L., G.J. Russell, J.L. Gittleman and T.M. Brooks, 1995. The future of biodiversity. Science, 269:347–350.–UNEP, 2003. Review of the interlinkages between biological diversity and climate change, and advice on the integration of biodiversity considerations into the implementation of the United Nations Framework Convention on Climate Change and its Kyoto Protocol. UNEP/CBD/SBSTTA/9/11.
- ↑ CAFF, 2001. Arctic Flora and Fauna: Status and Conservation. Conservation of Arctic Flora and Fauna, Helsinki, 272pp.–IPCC, 2001a. Climate Change 2001: Synthesis Report. A Contribution of Working Groups I, II and III to the Third Assessment Report of the Intergovernmental Panel on Climate Change. R.T. Watson and Core Writing Team. Cambridge University Press, 398pp.–Pimm, S.L., G.J. Russell, J.L. Gittleman and T.M. Brooks, 1995. The future of biodiversity. Science, 269:347–350.–UNEP, 2003. Review of the interlinkages between biological diversity and climate change, and advice on the integration of biodiversity considerations into the implementation of the United Nations Framework Convention on Climate Change and its Kyoto Protocol. UNEP/CBD/SBSTTA/9/11.
- ↑ UNEP, 2003. Review of the interlinkages between biological diversity and climate change, and advice on the integration of biodiversity considerations into the implementation of the United Nations Framework Convention on Climate Change and its Kyoto Protocol. UNEP/CBD/SBSTTA/9/11.
- ↑ Chapin, F.S. III, E.S. Zavaleta, V.T. Eviner, R.L. Naylor, P.M. Vitousek, H.L. Reynolds, D.U. Hooper, S. Lavorel, O.E. Sala, S.E. Hobbie, M.C. Mack and S. Dìaz, 2000. Consequences of changing biodiversity. Nature, 405:234–242.
- ↑ UNEP, 2003. Review of the interlinkages between biological diversity and climate change, and advice on the integration of biodiversity considerations into the implementation of the United Nations Framework Convention on Climate Change and its Kyoto Protocol. UNEP/CBD/SBSTTA/9/11.
- ↑ UNEP, 2003. Review of the interlinkages between biological diversity and climate change, and advice on the integration of biodiversity considerations into the implementation of the United Nations Framework Convention on Climate Change and its Kyoto Protocol. UNEP/CBD/SBSTTA/9/11.
- ↑ Oswood, M.W., 1997. Streams and rivers of Alaska. In: A.M. Milner and M.W. Oswood (eds.). Freshwaters of Alaska: Ecological Syntheses. Ecological Studies 119:331–356.
- ↑ Tang, E.P.Y. and W.F. Vincent, 1999. Strategies of thermal adaptation by high-latitude cyanobacteria. New Phytologist, 142:315–323.–Tang, E.P.Y., R. Tremblay and W.F. Vincent, 1997. Cyanobacterial dominance of polar freshwater ecosystems: Are high latitude mat-formers adapted to low temperature– Journal of Phycology, 33:171–181.
- ↑ Danks, H.V., 1992. Arctic insects as indicators of environmental change. Arctic, 45:159–166.
- ↑ Scrimgeour, G.J., T.D. Prowse, J.M. Culp and P.A. Chambers, 1994. Ecological effects of river ice break-up: a review and perspective. Freshwater Biology, 32:261–275.
- ↑ Prowse, T.D. and J.M. Culp, 2003. Ice break-up: a neglected factor in river ecology. Canadian Journal of Civil Engineering, 30:145–155.
- ↑ Sandstrom, S.J., 1995. The effect of overwintering site temperature on energy allocation and life history characteristics of anadromous female Dolly Varden char (Salvelinus malma), from northwestern Canada. M.Sc. Thesis, University of Manitoba, 161pp.
- ↑ Oswood, M.W., A.M. Milner and J.G. Irons III, 1992. Climate change and Alaskan rivers and streams. In: P. Firth and S.G. Fisher (eds.). Global Climate Change and Freshwater Ecosystems, pp. 192–210, Springer-Verlag.
- ↑ Krupnik, I. and D. Jolly (eds.), 2002. The Earth is Faster Now: Indigenous Observations of Arctic Environmental Change. Arctic Research Consortium of the United States, Fairbanks, Alaska, 384pp.
- ↑ Alexander, V., D.W. Stanley, R.J. Daley and C.P. McRoy, 1980. Primary producers. In: J.E. Hobbie (ed.). Limnology of Tundra Ponds: Barrow, Alaska, pp. 179–250. Dowden, Hutchinson and Ross.
- ↑ Thorpe, W., 1986. A Review of the Literature and Miscellaneous Other Parameters Relating to Water Levels in the Peace-Athabasca Delta Particularly with Respect to the Effect on Muskrat Numbers. Environment Canada.
- ↑ Wilson, C.C., P.D.N. Hebert, J.D. Reist and J.B. Dempson, 1996. Phylogeography and postglacial dispersion of arctic charr (Salvelinus alpinus L.) in North America. Molecular Ecology, 5:187–198.
- ↑ Craig, P.C. and P.J. McCart, 1975. Classification of stream types in Beaufort Sea drainages between Prudhoe Bay, Alaska and the Mackenzie Delta, N.W.T., Canada. Arctic and Alpine Research, 7:183–198.
- ↑ Deegan, L.A., H.E. Golden, C.J. Harvey and B.J. Peterson, 1999. Influence of environmental variability on the growth of age-0 and adult Arctic grayling. Transactions of the American Fisheries Society, 128:1163–1175.–Hershey, A.E., W.B. Bowden, L.A. Deegan, J.E. Hobbie, B.J. Peterson, G.W. Kipphut, G.W. Kling, M.A. Lock, R.W. Merritt, M.C. Miller, J.R. Vestal and J.A. Schuldt, 1997.The Kuparuk River: a long-term study of biological and chemical processes in an arctic river. In: A.M. Milner and M.W. Oswood (eds.). Freshwaters of Alaska: Ecological Syntheses. Ecological Studies 119:107–130.
- ↑ Peterson, B.J., L. Deegan, J. Helfrich, J.E. Hobbie, M. Hullar, B. Moller, T.E. Ford, A. Hershey, A. Hiltner, G. Kipphut, M.A. Lock, D.M. Fiebig, V. McKinley, M.C. Miller, J.R. Vestal, J. Robie, R. Ventullo and G. Volk, 1993. Biological responses of a tundra river to fertilization. Ecology, 74:653–672.
- ↑ Harvey, C.J., B.J. Peterson, W.B. Bowden, A.E. Hershey, M.C. Miller, L.A. Deegan and J.C. Finlay, 1998. Biological responses to fertilization of Oksrukuyik Creek, a tundra stream. Journal of the North American Benthological Society, 17:190–209.–Peterson, B.J., L. Deegan, J. Helfrich, J.E. Hobbie, M. Hullar, B. Moller, T.E. Ford, A. Hershey, A. Hiltner, G. Kipphut, M.A. Lock, D.M. Fiebig, V. McKinley, M.C. Miller, J.R. Vestal, J. Robie, R. Ventullo and G. Volk, 1993. Biological responses of a tundra river to fertilization. Ecology, 74:653–672.
- ↑ Bowden, W.B., J.C. Finlay and P.E. Maloney, 1994. Long-term effects of PO4 fertilization on the distribution of bryophytes in an arctic river. Freshwater Biology, 32:445–454.
- ↑ Bowden, W.B., D.B. Arscott, D. Pappathanasi, J.C. Finlay, J.M. Glime, J. LeCroix, C.-L. Liao, A.E. Hershey, T. Lampella, B.J. Peterson, W.Wollheim, K. Slavik, B. Shelley, M. Chesterton, J.A. Lachance, R. Le Blanc, A. Steinman and A. Suren, 1999. Roles of bryophytes in stream ecosystems. Journal of the North American Benthological Society, 18:151–184.
- ↑ Vincent, W.F. and J.E. Hobbie, 2000. Ecology of Arctic lakes and rivers. In: M. Nuttall and T.V. Callaghan (eds.). The Arctic: Environment, People, Policy, pp. 197–231. Harwood Academic Press.
- ↑ Kalff, J., 2002. Limnology: Inland Water Ecosystems. Prentice Hall, 592pp.
- ↑ Kalff, J., 2002. Limnology: Inland Water Ecosystems. Prentice Hall, 592pp.
- ↑ Pienitz, R., J.P. Smol and H.J.B. Birks, 1995. Assessment of freshwater diatoms as quantitative indicators of past climate change in the Yukon and Northwest Territories, Canada. Journal of Paleolimnology, 13:21–49.
- ↑ Fallu, M.-A. and R. Pienitz, 1999. Diatomées lacustres de Jamésie-Hudsonie (Québec) et modèle de reconstitution des concentrations de carbone organique dissous. Ecoscience, 6:603–620.–Lotter, A.F., R. Pienitz and R. Schmidt, 1999. Diatoms as indicators of environmental change near Arctic and Alpine treeline. In: E.F. Stoermer and J.P. Smol (eds.). The Diatoms: Applications to the Environmental and Earth Sciences, pp. 205–226, Cambridge University Press.
- ↑ Beisner, B.E., E. McCauley and F.J. Wrona, 1997. The influence of temperature and food chain length on plankton predator-prey dynamics. Canadian Journal of Fisheries and Aquatic Sciences, 54:586–595.–McCauley, E. and W.W. Murdoch, 1987. Cyclic and stable populations: plankton as paradigm. The American Naturalist, 129:97–121.
- ↑ Beisner, B.E., E. McCauley and F.J. Wrona, 1996. Temperature-mediated dynamics of planktonic food chains: the effect of an invertebrate carnivore. Freshwater Biology, 35:219–231.–Beisner, B.E., E. McCauley and F.J. Wrona, 1997.The influence of temperature and food chain length on plankton predator-prey dynamics. Canadian Journal of Fisheries and Aquatic Sciences, 54:586–595.
- ↑ McDonald, M.E., A.E. Hershey and M.C. Miller, 1996. Global warming impacts on lake trout in Arctic lakes. Limnology and Oceanography, 41:1102–1108.
- ↑ Hobbie, J.E., B.J. Peterson, N. Bettez, L. Deegan, W.J. O'Brien, G.W. Kling, G.W. Kipphut, W.B. Bowden and A.E. Hershey, 1999. Impact of global change on the biogeochemistry and ecosystems of an arctic freshwater system. Polar Research, 18:207–214.
- ↑ Stross, R.G., M.C. Miller and R.J. Daley, 1980. Zooplankton. In: J.E. Hobbie (ed.). Limnology of Tundra Ponds: Barrow, Alaska, pp. 251–296. Dowden, Hutchinson and Ross.
- ↑ Rouse, W.R., M.S.V. Douglas, R.E. Hecky, A.E. Hershey, G.W. Kling, L. Lesack, P. Marsh, M. McDonald, B.J. Nicholson, N.T. Roulet and J.P. Smol, 1997. Effects of climate change on the freshwaters of Arctic and subarctic North America. Hydrological Processes, 11:873–902.
- ↑ Carpenter, S.R., S.G. Fisher, N.B. Grimm and J.F. Kitchell, 1992. Global change and freshwater ecosystems. Annual Review of Ecology and Systematics, 23:119–139.–Goyke, A.P. and A.E. Hershey, 1992. Effects of fish predation on larval chironomid (Diptera, Chironomidae) communities in an arctic ecosystem. Hydrobiologia, 240:203–212.–Hanson, K.L., A.E. Hershey and M.E. McDonald, 1992. A comparison of slimy sculpin (Cottus cognatus) populations in arctic lakes with and without piscivorous predators. Hydrobiologia, 240:189–202.–Hershey, A.E., 1990. Snail populations in arctic lakes: competition mediated by predation. Oecologia, 82:26–32.–Jeppesen, E., J.P. Jensen, C. Jensen, B. Faafeng, D.O. Hessen, M. Søndergaard, T. Lauridsen, P. Brettum and K. Christoffersen, 2003. The impact of nutrient state and lake depth on top-down control in the pelagic zone of lakes: a study of 466 lakes from the temperate zone to the Arctic. Ecosystems, 6:313–325.–O'Brien, W.J., A.E. Hershey, J.E Hobbie, M.A. Hullar, G.W. Kipphut, M.C. Miller, B. Moller and J.R. Vestal, 1992. Control mechanisms of arctic lake ecosystems: a limnocorral experiment. Hydrobiologia, 240:143–188.
- ↑ Flanagan, K., E. McCauley, F. Wrona and T.D. Prowse, 2003. Climate change: the potential for latitudinal effects on algal biomass in aquatic ecosystems. Canadian Journal of Fisheries and Aquatic Sciences, 60: 635–639.
- ↑ Golden, H.E. and L.A. Deegan, 1998. The trophic interactions of young arctic grayling (Thymallus arcticus) in an Arctic tundra stream. Freshwater Biology, 39:637–648.–Hansson, L.-A., 1992. The role of food chain composition and nutrient availability in shaping algal biomass development. Ecology, 73:241–247.–McQueen, D.J., M.R.S. Johannes, J.R. Post, T.J. Stewart and D.R.S. Lean, 1989. bottom:up and top-down impacts on freshwater pelagic community structure. Ecological Monographs, 59:289–309.
- ↑ McQueen, D.J., M.R.S. Johannes, J.R. Post, T.J. Stewart and D.R.S. Lean, 1989. bottom:up and top-down impacts on freshwater pelagic community structure. Ecological Monographs, 59:289–309.
- ↑ Flanagan, K., E. McCauley, F. Wrona and T.D. Prowse, 2003. Climate change: the potential for latitudinal effects on algal biomass in aquatic ecosystems. Canadian Journal of Fisheries and Aquatic Sciences, 60:635–639.
- ↑ Golden, H.E. and L.A. Deegan, 1998. The trophic interactions of young arctic grayling (Thymallus arcticus) in an Arctic tundra stream. Freshwater Biology, 39:637–648.
- ↑ Hobbie, J.E., B.J. Peterson, N. Bettez, L. Deegan, W.J. O'Brien, G.W. Kling, G.W. Kipphut, W.B. Bowden and A.E. Hershey, 1999. Impact of global change on the biogeochemistry and ecosystems of an arctic freshwater system. Polar Research, 18:207–214.–Laurion, I., W.F. Vincent and D.R.S. Lean, 1997. Underwater ultraviolet radiation: development of spectral models for northern high latitude lakes. Photochemistry and Photobiology, 65:107–114.–Rouse, W.R., M.S.V. Douglas, R.E. Hecky, A.E. Hershey, G.W. Kling, L. Lesack, P. Marsh, M. McDonald, B.J. Nicholson, N.T. Roulet and J.P. Smol, 1997. Effects of climate change on the freshwaters of Arctic and subarctic North America. Hydrological Processes, 11:873–902.–Vincent, W.F. and J.E. Hobbie, 2000. Ecology of Arctic lakes and rivers. In: M. Nuttall and T.V. Callaghan (eds.). The Arctic: Environment, People, Policy, pp. 197–231. Harwood Academic Press.
- ↑ Rae, R. and W.F. Vincent, 1998b. Phytoplankton production in subarctic lake and river ecosystems: development of a photosynthesis-temperature-irradiance model. Journal of Plankton Research, 20:1293–1312.
- ↑ Brylinsky, M. and K.H. Mann, 1973. An analysis of factors governing productivity in lakes and reservoirs. Limnology and Oceanography, 18:1–14.
- ↑ Flanagan, K., E. McCauley, F. Wrona and T.D. Prowse, 2003. Climate change: the potential for latitudinal effects on algal biomass in aquatic ecosystems. Canadian Journal of Fisheries and Aquatic Sciences, 60:635–639.
- ↑ Shortreed, K.S. and J.G. Stockner, 1986. Trophic status of 19 subarctic lakes in the Yukon Territory. Canadian Journal of Fisheries and Aquatic Sciences, 43:797–805.
- ↑ Flanagan, K., E. McCauley, F. Wrona and T.D. Prowse, 2003. Climate change: the potential for latitudinal effects on algal biomass in aquatic ecosystems. Canadian Journal of Fisheries and Aquatic Sciences, 60:635–639.
- ↑ Watson, S.B., E. McCauley and J.A. Downing, 1997. Patterns in phytoplankton taxonomic composition across temperate lakes of differing nutrient status. Limnology and Oceanography, 42:487–495.
- ↑ Flanagan, K., E. McCauley, F. Wrona and T.D. Prowse, 2003. Climate change: the potential for latitudinal effects on algal biomass in aquatic ecosystems. Canadian Journal of Fisheries and Aquatic Sciences, 60:635–639.
- ↑ Meltofte, H. and H.Thing (eds.), 1997. Zackenberg Ecological Research Operations, 2nd Annual Report, 1996. Danish PolarCenter, Ministry of Research and Information Technology, Copenhagen, 80pp.
- ↑ Christoffersen, K. and E. Jeppesen, 2000. Lake monitoring. In: K. Caning and M. Rasch (eds.). Zackenberg Ecological Research Operations, 5th Annual Report, 1999, pp. 43–46. Danish Polar Center, Ministry of Research and Information Technology, Copenhagen.
- ↑ Rigler, F.H., 1978. Limnology in the high Arctic: a case study of Char Lake.Verheissungen der Internationale Vereinigung der gesamten Limnologie, 20:127–140.
- ↑ Data provided by BioBasis
- ↑ Hansson, L.-A., 1992. The role of food chain composition and nutrient availability in shaping algal biomass development. Ecology, 73:241–247.
- ↑ Shortreed, K.S. and J.G. Stockner, 1986. Trophic status of 19 subarctic lakes in the Yukon Territory. Canadian Journal of Fisheries and Aquatic Sciences, 43:797–805.
- ↑ Pienitz, R. and W.F. Vincent, 2000. Effect of climate change relative to ozone depletion on UV exposure in subarctic lakes. Nature, 404:484–487.
- ↑ Hecky, R.E. and S.J. Guildford, 1984. Primary productivity of Southern Indian Lake before, during and after impoundment and Churchill River Diversion. Canadian Journal of Fisheries and Aquatic Sciences, 41:591–604.
- ↑ Stanley, D.W., 1976. Productivity of epipelic algae in tundra ponds and a lake near Barrow, Alaska. Ecology, 57:1015–1024.
- ↑ Vincent, W.F. and J.E. Hobbie, 2000. Ecology of Arctic lakes and rivers. In: M. Nuttall and T.V. Callaghan (eds.).The Arctic: Environment, People, Policy, pp. 197–231. Harwood Academic Press.
- ↑ O'Brien, W.J., A.E. Hershey, J.E Hobbie, M.A. Hullar, G.W. Kipphut, M.C. Miller, B. Moller and J.R. Vestal, 1992. Control mechanisms of arctic lake ecosystems: a limnocorral experiment. Hydrobiologia, 240:143–188.
- ↑ Rublee, P., 1992. Community structure and bottom:up regulation of heterotrophic microplankton in arctic LTER lakes. Hydrobiologia, 240:133–142.
- ↑ Kling, G.W., J. O'Brien, M.C. Miller and A.E. Hershey, 1992a. The biogeochemistry and zoogeography of lakes and rivers in arctic Alaska. Hydrobiologia, 240:1–14.
- ↑ Bahr, M., J.E. Hobbie and M.L. Sogin, 1996. Bacterial diversity in an Arctic lake – a freshwater SAR11 cluster. Aquatic Microbial Ecology, 11:271–277.
- ↑ Crump, B.C., G.W. Kling, M. Bahr and J.E. Hobbie, 2003. Bacterioplankton community shifts in an Arctic lake correlate with seasonal changes in organic matter source. Applied and Environmental Microbiology, 69:2253–2268.
- ↑ O'Brien, W.J., A.E. Hershey, J.E. Hobbie, M.A. Hullar, G.W. Kipphut, M.C. Miller, B. Moller and J.R.Vestal, 1992. Control mechanisms of arctic lake ecosystems: a limnocorral experiment. Hydrobiologia, 240:143–188.
- ↑ Bettez, N.D., P.A. Rublee, J. O'Brien and M.C. Miller, 2002. Changes in abundance, composition and controls within the plankton of a fertilised arctic lake. Freshwater Biology, 47:303–311.
- ↑ Groisman, P.Y., T.R. Karl and R.W. Knight, 1994. Observed impact of snow cover on the heat balance and the rise of continental spring temperatures. Science, 263:198–200.
- ↑ Turetsky, M.R., R.K. Wieder and D.H. Vitt, 2002. Boreal peatland C fluxes under varying permafrost regimes. Soil Biology and Biochemistry, 34:907–912.
- ↑ Panikov, N.S. and S.N. Dedysh, 2000. Cold season CH4 and CO2 emission from boreal peat bogs (West Siberia): winter fluxes and thaw activation dynamics. Global Biogeochemical Cycles, 14:1071–1080.
- ↑ Friborg, T., T.R. Christensen and H. Soegaard, 1997. Rapid response of greenhouse gas emission to early spring thaw in a subarctic mire as shown by micrometeorological techniques. Geophysical Research Letters, 24:3061–3064.–Svensson, B.H., T.R. Christensen, E. Johansson and M. Öquist, 1999. Interdecadal changes in CO2 and CH4 fluxes of a subarctic mire: Stordalen revisited after 20 years. Oikos, 85:22–30.
- ↑ Gignac, L.D. and D.H. Vitt, 1994. Responses of northern peatlands to climatic change, effects on bryophytes. Journal of the Hattori Botanical Laboratory, 75:119–132.–Nicholson, B.J. and L.D. Gignac, 1995. Ecotype dimensions of peatland bryophyte indicator species along gradients in the Mackenzie River Basin, Canada.The Bryologist, 98:437–451.
- ↑ Gajewski, K., R. Vance, M. Sawada, I. Fung, L.D. Gignac, L. Halsey, J. John, P. Maisongrande, P. Mandell, P.J. Mudie, P.J.H. Richard, A.G. Sherin, J. Soroko and D.H. Vitt, 2000. The climate of North America and adjacent ocean waters ca. 6 ka. Canadian Journal of Earth Sciences, 37:661–681.–Vardy, S.R., B.G. Warner and R. Aravena, 1997. Holocene climate effects on the development of a peatland on the Tuktoyaktuk Peninsula, Northwest Territories. Quaternary Research, 47:90–104.
- ↑ Robinson, S.D. and T.R. Moore, 2000.The influence of permafrost and fire upon carbon accumulation in high boreal peatlands, Northwest Territories, Canada. Arctic, Antarctic, and Alpine Research, 32:155–166.–Turetsky, M.R., R.K. Wieder, C.J. Williams and D.H. Vitt, 2000. Organic matter accumulation, peat chemistry, and permafrost melting in peatlands of boreal Alberta. Ecoscience, 7:379–392.–Vitt, D.H., L.A. Halsey and S.C. Zoltai, 2000. The changing landscape of Canada's western boreal forest: the current dynamics of permafrost. Canadian Journal of Forest Research, 30:283–287.
- ↑ Robinson, S.D. and T.R. Moore, 2000. The influence of permafrost and fire upon carbon accumulation in high boreal peatlands, Northwest Territories, Canada. Arctic, Antarctic, and Alpine Research, 32:155–166.–Turetsky, M.R., R.K. Wieder and D.H. Vitt, 2002. Boreal peatland C fluxes under varying permafrost regimes. Soil Biology and Biochemistry, 34:907–912.
- ↑ Laing, T.E., K.M. Rühland and J.P. Smol, 1999. Past environmental and climatic changes related to tree-line shifts inferred from fossil diatoms from a lake near the Lena River Delta, Siberia. The Holocene, 9:547–557.
- ↑ Christensen, T.R., T. Friborg, M. Sommerkorn, J. Kaplan, L. Illeris, H. Soegaard, C. Nordstroem and S. Jonasson, 2000. Trace gas exchange in a high-arctic valley. 1. Variations in CO2 and CH4 flux between tundra vegetation types. Global Biogeochemical Cycles, 14:701–713.
- ↑ Laurila, T., H. Soegaard, C.R. Lloyd, M. Aurela, J.-P. Tuovinen and C. Nordstroem, 2001. Seasonal variations of net CO2 exchange in European Arctic ecosystems.Theoretical and Applied Climatology, 70:183–201.
- ↑ Niemi, R., P.J. Martikainen, J. Silvola, A. Wulff, S. Turtola and T. Holopainen, 2002. Elevated UV-B radiation alters fluxes of methane and carbon dioxide in peatland microcosms. Global Change Biology, 8:361-371.
- ↑ Funk, D.W., E.R. Pullman, K.M. Peterson, P.M. Crill and W.D. Billings, 1994. Influence of water table on carbon dioxide, carbon monoxide, and methane fluxes from taiga bog microcosms. Global Biogeochemical Cycles, 8:271–278.
- ↑ Aurela, M., T. Laurila and J.-P. Tuovinen, 2001. Seasonal CO2 balances of a subarctic mire. Journal of Geophysical Research, 106(D2):1623–1637.
- ↑ Minkkinen, K., R. Korhonen, I. Savolainen and J. Laine, 2002. Carbon balance and radiative forcing of Finnish peatlands 1900–2100 - the impact of forestry drainage. Global Change Biology, 8:785–799.
- ↑ Joabsson, A. and T.R. Christensen, 2001. Methane emissions from wetlands and their relationship with vascular plants: an Arctic example. Global Change Biology, 7:919–932.
- ↑ Moore, T.R. and N.T. Roulet, 1993. Methane flux: water table relations in northern wetlands. Geophysical Research Letters, 20:587–590.
- ↑ Liblik, L.K., T.R. Moore, J.L. Bubier and S.D. Robinson, 1997. Methane emissions from wetlands in the zone of discontinuous permafrost: Fort Simpson, Northwest Territories, Canada. Global Biogeochemical Cycles, 11:485–494.
- ↑ Hargreaves, K.J., D. Fowler, C.E.R. Pitcairn and M. Aurela, 2001. Annual methane emission from Finnish mires estimated from eddy covariance campaign measurements.Theoretical and Applied Climatology, 70:203–213.
- ↑ Funk, D.W., E.R. Pullman, K.M. Peterson, P.M. Crill and W.D. Billings, 1994. Influence of water table on carbon dioxide, carbon monoxide, and methane fluxes from taiga bog microcosms. Global Biogeochemical Cycles, 8:271–278.
- ↑ Gorham, E., 1991. Northern peatlands: role in the carbon cycle and probable responses to climatic warming. Ecological Applications, 1:182–195.–Moore, T.R., N.T. Roulet and J.M. Waddington, 1998. Uncertainty in predicting the effect of climatic change on the carbon cycling of Canadian peatlands. Climatic Change, 40:229–245.
- ↑ Kling, G.W., G.W. Kipphut, M.M. Miller and W.J. O'Brien, 2000. Integration of lakes and streams in a landscape perspective: the importance of material processing on spatial patterns and temporal coherence. Freshwater Biology, 43:477–497.
- ↑ Kling, G.W., G.W. Kipphut and M.C. Miller, 1992b.The flux of CO2 and CH4 from lakes and rivers in arctic Alaska. Hydrobiologia, 240:23–36.–Schindler, D.E., S.R. Carpenter, J.J. Cole, J.F. Kitchell and M.L. Pace, 1997. Influence of food web structure on carbon exchange between lakes and the atmosphere. Science, 277:248–251.
- ↑ Kling, G.W., G.W. Kipphut and M.C. Miller, 1992b.The flux of CO2 and CH4 from lakes and rivers in arctic Alaska. Hydrobiologia, 240:23–36.
- ↑ Kling, G.W., G.W. Kipphut and M.C. Miller, 1992b.The flux of CO2 and CH4 from lakes and rivers in arctic Alaska. Hydrobiologia, 240:23–36.–Schindler, D.E., S.R. Carpenter, J.J. Cole, J.F. Kitchell and M.L. Pace, 1997. Influence of food web structure on carbon exchange between lakes and the atmosphere. Science, 277:248–251.
- ↑ Ramlal, P.S., R.H. Hesslein, R.E. Hecky, E.J. Fee, J.W.M. Rudd and S.J. Guildford, 1994.The organic carbon budget of a shallow arctic tundra lake on the Tuktoyaktuk Peninsula, NWT, Canada: Arctic lake carbon budget. Biogeochemistry, 24:145–172.
- ↑ Zimov, S.A., Y.V. Voropaev, I.P. Semiletov, S.P. Davidov, S.F. Prosiannikov, F.S. Chapin III, M.C. Chapin, S. Trumbore and S. Tyler, 1997. North Siberian lakes: A methane source fueled by Pleistocene carbon. Science, 277:800–802.
- ↑ Semiletov, I.P., 2001. Atmospheric methane and carbon dioxide in the arctic. In: I.P. Semiletov (ed.). Proceedings of the Arctic Regional Centre/V.I. Il–ichev Pacific Oceanological Institute, Volume 3. Hydrochemistry and Greenhouse Gas, pp. 127–164. Dalnauka, Vladivostok.–Zimov, S.A., Y.V. Voropaev, S.P. Davidov, G.M. Zimova, A.I. Davidova, F.S. Chapin III and M.C. Chapin, 2001. Flux of methane from north Siberian aquatic systems: influence on atmospheric methane. In: R. Paepe, V. Melnikov, E. Van Overloop and V.D. Gorokhov (eds.). Permafrost Response on Economic Development, Environmental Security and Natural Resources, pp. 511–524.