Information required to project responses of arctic fish
This is Section 8.5.1 of the Arctic Climate Impact Assessment
Lead Authors: Frederick J.Wrona,Terry D. Prowse, James D. Reist; Contributing Authors: Richard Beamish, John J. Gibson, John Hobbie, Erik Jeppesen, Jackie King, Guenter Koeck, Atte Korhola, Lucie Lévesque, Robie Macdonald, Michael Power,Vladimir Skvortsov,Warwick Vincent; Consulting Authors: Robert Clark, Brian Dempson, David Lean, Hannu Lehtonen, Sofia Perin, Richard Pienitz, Milla Rautio, John Smol, Ross Tallman, Alexander Zhulidov
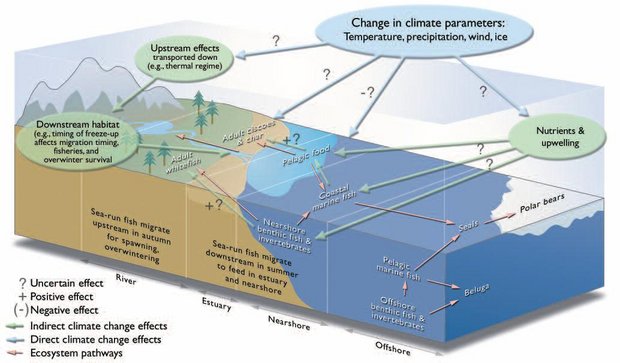
Implicit in much of the previous text is the linkage between atmospheric climate parameters and habitat parameters present in aquatic ecosystems, and the linkage of these to effects manifested in organisms and populations. It follows from this logic that changes in climate regimes, however they may manifest, will only indirectly affect aquatic organisms of interest. That is, the aquatic environment itself will be directly affected by changes in climate, but will modify and then transmit the influences in some fashion. Thus, for example, substantive shifts in atmospheric temperature regimes will affect water temperatures, but given the density differences between water and air and the influence of hydrodynamic factors, the effects on aquatic systems will be modified to some degree. In turn, changes in atmospheric parameters will have indirect effects on biota present in aquatic systems and thus may be ameliorated or partially buffered (e.g., thermal extremes or seasonal timing shifted). In some instances, however, climate change effects may be magnified or exacerbated, increasing the multiplicity of possible outcomes resulting from these changes. For example, stream networks amplify many environmental signals that occur at the watershed level, and that are concentrated in the stream channel[1]. This added level of complexity and uncertainty in the magnitude and direction of climate change manifestations in arctic freshwater ecosystems is not as acute for terrestrial environments. It results in greater uncertainty in projecting potential impacts on aquatic organisms. Figure 8.16 provides an example of the logical associations and direct and indirect effects of climate parameters on anadromous fish and the various aquatic environments used.
Contents
Fish and climate parameters (8.5.1.1)
The Arctic as defined in this chapter (Fig. 8.2) includes high-, low-, and subarctic areas defined by climate, geography, and physical characteristics. In addition, many areas included in this assessment (e.g., southern Alaska, the southern Northwest Territories, northern Scandinavia, and Russia) are significantly influenced by nearby southern maritime environments and/or large northward-flowing rivers. This proximity ameliorates local climatic regimes, resulting in more northerly distributions of aquatic taxa than would otherwise occur based strictly on latitudinal position. Moreover, the Arctic includes many different climatological zones. Given that the distribution of many freshwater and anadromous fish species is controlled or significantly influenced either directly or indirectly by climate variables (particularly temperature), it follows that primary associations of fish distribution with climate variables will be important.
Fish are ectotherms, thus, for the most part, their body temperature is governed by that of the surrounding waters. In addition, individual fish species can behaviorally choose specific thermal preferenda (preferred optimal temperatures[2]) at which physiological processes are optimal (i.e., greatest net benefit is achieved for the individual). This is typically a thermal range that may be fairly narrow; temperatures outside this are suboptimal (i.e., net benefit is still attained but it is not the greatest possible), grading to detrimental (i.e., non-lethal but net energy is expended while in such conditions) and ultimately to lethal conditions (i.e., death ensues after some level of exposure). Furthermore, within a species, local northern populations often have such preferenda set lower than do southern representatives, which presumably represents differential adaptation to local conditions. In addition, individual life stages (e.g., egg, alevin, juvenile, adult) differ in their thermal preferenda linked to optimizing criteria specific to their developmental stage. For most species, only limited understanding of such thermal optima is available, and typically only for some life stages of southern species. Fish control body temperatures behaviorally, sensing and moving into appropriate, or from inappropriate, zones[3]. Aquatic thermal regimes are spatially and temporally heterogeneous and availability of water at the preferred temperature may be limited, making it an important resource for which competition may ensue. This may be particularly important in species found in Alaskan and Yukon North Slope rivers (e.g., Dolly Varden and Arctic grayling) during winter, when physical habitat (Habitat selection) is limited due to rivers (River and lake ice in the Arctic) freezing to the bottom over long reaches[4]. Thus, the thermal niche of individual fish species can be defined.
Magnuson et al.[5] grouped temperate species into three thermal guilds defined by thermal niches: Warmwater (preferred summer temperatures centered upon 27–31°C), Coolwater (21–25°C), and Coldwater (11–15°C). Following this approach, Reist[6] defined an Arctic Guild as fish distributed wholly or primarily in northern areas and adapted to relatively colder waters (<10°C) and related aspects of the habitat such as short growing seasons, extensive ice presence, and long periods of darkness. Freshwater fishes occurring within the geographic definition of the Arctic as used here represent all of these guilds (Box 8.6), however, those of the Warmwater Guild tend to be present only along the southern margins of arctic waters, often associated with local climatic amelioration resulting from inputs from nearby maritime areas or northward-flowing rivers. Some of these guilds can be further subdivided based upon the nature of the fish distribution. Within the generalities discussed below, the impacts of climate change will be species- and ecosystem-specific, thus the following should be viewed as a range of possibilities only. In addition, although thermal regimes are emphasized in this discussion, the influence of other climate parameters may be equally or more important to specific species in particular areas or at particular times during life.
Species of the Arctic Guild have their center of distribution in the Arctic with the southern limits defined by, for example, high [[temperature]s] and associated ecological factors including competition from southern fish species. Fish such as broad whitefish (Coregonus nasus), Arctic cisco, and many char taxa are examples of Arctic Guild species. The pervasive and ultimate impacts of climate change upon such species are likely to be negative. These impacts generally will appear as range contractions northward driven by thermal warming that exceeds preferences or tolerances; related habitat changes; and/or increased competition, predation, or disease resulting from southern taxa extending their range northward, possibly preceded by local reductions in growth, productivity, and perhaps abundance. Many of these effects will possibly be driven or exacerbated by shifts in the life history of some species (e.g., from anadromy to freshwater only). Other than conceptual summaries, no detailed research has been conducted to outline such impacts for most fish species of this guild.
|
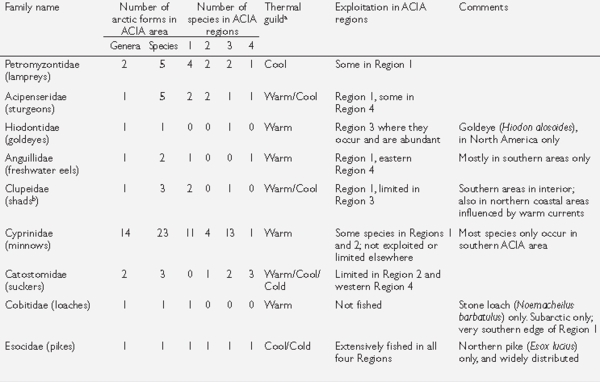
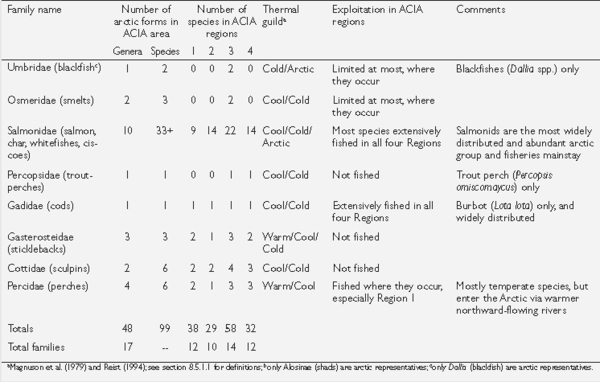
Box 8.6. Freshwater and diadromous fishes of the Arctic There are approximately 99 species in 48 genera of freshwater and diadromous (i.e., anadromous or catadromous forms moving between fresh and marine waters) fish present in the Arctic as defined in this chapter (Fig. 8.2).These represent 17 families (see table). Ninety-nine species is a conservative estimate because some groups (e.g., chars and whitefishes) in fact contain complexes of incompletely resolved species. Many species are also represented by local polymorphic forms that biologically act as species (e.g., four morphs of Arctic char in Thingvallavatn, Iceland).The most species-rich family is the Salmonidae with more than 33 species present, most of which are important in fisheries.The next most species-rich family is the Cyprinidae with 23 species, few of which are fished generally, although some may be fished locally. All remaining families have six or fewer species, and five families are represented in the Arctic by a single species.These generalities hold true for the individual ACIA regions as well. All of the families represented in the Arctic are also present in lower-latitude temperate and subtemperate regions. Most have a southern center of distribution, as do many of their associated species[7]. Individual species may be confined to the Arctic, or may penetrate northward from subarctic areas to varying degrees. Substantive differences in the number of species present are apparent between the ACIA regions. Region 3 (unglaciated Beringia and the western Canadian Arctic) contains 58 named taxa, followed by Region 1 (Arctic Europe and Russia) with 38, while Regions 2 (Siberia) and 4 (eastern North America) are about equal at 29 and 32, respectively. This probably represents a combination of historical effects (e.g., glacial events, postglacial recolonization routes and access) as well as present-day influences such as local climate, habitat diversity, and ecological processes (e.g., competition and predation). Arctic char is the only species that is truly holarctic, being present on all landmasses in all ACIA regions, occurring the farthest north to the extremes of land distribution (~84º N), and also exhibiting the widest latitudinal range (about 40 degrees) of all true arctic species (i.e., south in suitable lakes to ~45º N). A few additional species are distributed almost completely across the Holarctic but are absent from one or more areas within an ACIA region (e.g., burbot with ~75% of a complete circumpolar distribution; northern pike with ~85%; lake whitefish, European whitefish, and Siberian whitefish (Coregonus pidschian) with ~85%; and ninespine stickleback with ~90%). With the exception of the stickleback, all are fished extensively where they occur, representing the mainstays of food fisheries for northern peoples and supporting significant commercial fisheries in most areas.These species are often the only ones present in extremely remote areas, inland areas, and higher-latitude areas, and thus are vital for local fisheries. Where they are regionally present, many other species are exploited to a greater or lesser degree. aMagnuson et al.{{ref|8b} and Reist{{ref|8c); see Fish and Climate Parameters for definitions; bonly Alosinae (shads) are arctic representatives; conly Dallia (blackfish) are arctic representatives |
Fish that have distributions in the southern arctic are northern members of the Coldwater Guild. This group includes species such as the lake/European/Siberian whitefish complex and lake trout, which have narrow thermal tolerances but usually are widely distributed due to the availability of colder habitats in water bodies (e.g., deeper layers in lakes; higher-elevation reaches in [[stream]s][8]). Two distributional subtypes can be differentiated: those exhibiting a wide thermal tolerance (eurythermal) as implied, for example, by a wide latitudinal distribution often extending well outside the Arctic (e.g., lake whitefish – Coregonus clupeaformis); and those exhibiting a narrow thermal tolerance (stenothermal) implied by occupation of very narrow microhabitats (e.g., lake trout occupy deep lakes below thermoclines in the south but a much wider variety of coldwater habitats in the north) and/or narrow latitudinal distribution centered in northern areas (e.g., pond smelt – Hypomesus olidus). The overall impacts of arctic climate change on these two distributional subtypes are likely to be quite different. Thus, eurythermal species are likely to have the capacity for reasonably quick adaptation to changing climate and, all other things being equal, are likely to exhibit increases in growth, reproduction, and overall productivity. Such species are also likely to extend the northern edge of their distribution further northward where this is at present thermally limited, but this is likely to be a secondary, relatively small response. Conversely, stenothermal coldwater species are likely to experience generally negative impacts. Lake trout in northern lakes, for example, will possibly be forced into smaller volumes of suitable summer habitat below deeper lake thermoclines and will possibly have to enter such areas earlier in the season than at present. Subsequent impacts on such species are very likely to be negative as well. To some degree, northern members of the Coldwater Guild are likely to experience the same general impacts as described for arctic-guild species in the previous paragraph (i.e., reductions in productivity characteristics, increased stress, local extirpation, and/or range contractions). Similar to the arctic-guild species, little or no detailed research assessing impacts on northern coldwater-guild fishes has been conducted to date.
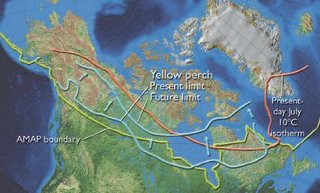 Fig. 8.19. The major biotic processes affecting the dynamics of a freshwater or anadromous arctic fish population and some of the aquatic abiotic environmental parameters that affect these processes. The tan ovals refer to the four major processes controlling fish production; these will shift as a result of climate change effects on the various components of the ecosystem[9].
Fig. 8.19. The major biotic processes affecting the dynamics of a freshwater or anadromous arctic fish population and some of the aquatic abiotic environmental parameters that affect these processes. The tan ovals refer to the four major processes controlling fish production; these will shift as a result of climate change effects on the various components of the ecosystem[9]. Coolwater Guild species (such as perches) have southern, temperate centers of distribution but range northward to the southern areas of the Arctic as defined herein; these include northern pike (Esox lucius), walleye (Sander vitreus), and yellow perch[10]. Like those of the Coldwater Guild, these species can also be differentiated into eurythermal and stenothermal species. For example, the perches have a wide latitudinal range and occupy a number of ecological situations extending outside temperate regions, and hence can be described as eurythermal. Northward range extensions of approximately two to eight degrees of latitude are projected for yellow perch in under a climate change scenario where annual mean [[temperature]s] increase by 4°C[11] (Fig. 8.17). Shuter and Post[12] found that the linkage between perch distribution and climate was indirect; that is, the first-order linkage was the direct dependency of overwinter survival (and related size at the end of the first summer of life) on food supply, which limited growth. The food supply, in turn, was dependent upon climate parameters. Alternatively, many northern minnows (e.g., northern redbelly dace – Chrosomus eos – in North America) and some coregonines (e.g., vendace – Coregonus albula – in Europe) are probably stenothermal, as implied by their limited latitudinal range and habitat associations. Range contraction along southern boundaries is likely for these species, initially manifested as contraction of distribution within the local landscapes, followed by northward retraction of the southern range limit. Because of their stenothermal tolerances, however, their northward extension is not likely to be as dramatic as that described for perch. To some degree, the presence of many of these species in the large northward-flowing arctic rivers such as the Lena, Mackenzie, Ob, and Yenisey is very likely to promote their northward penetration. The associated effects of heat transfer by such river systems will facilitate northward colonization by these species as well as eurythermal species also present in the systems. Knowledge of the association of ecological processes with climate parameters and research quantifying the potential impacts of climate change on coolwater-guild species, although inadequate overall, generally tends to be more comprehensive than for the previous two guilds, but is often focused upon southern populations. Hence, its applicability to arctic populations of the species may be limited.
Warmwater Guild species have their center of distribution well south of the Arctic. Those present in the Arctic as defined in this chapter are few in number and with few exceptions (some cyprinid species) are generally distributed only in the extreme southern portions of the ACIA regions. In many areas of the Arctic, a number of species of this guild are present in southerly areas immediately outside the boundary of the Arctic. Presumably, their northward limit is in most cases determined by present thermal and ecological regimes, especially in the large northward-flowing rivers of Siberia and the western Northwest Territories. As the effects of climate change increasingly ameliorate local limiting factors, species of this guild are very likely to extend their geographic ranges into the Arctic or, if already there, to more northerly locales.
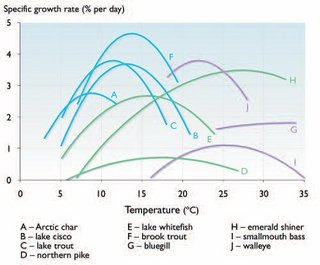 Fig. 8.18. Growth rates of fish species at varying temperatures determined from laboratory studies. Stenothermic northern species (e.g.,A, B, C, and F) are grouped towards the lower temperatures on the left, whereas mesothermic southern species (e.g., G, I, and J) are grouped towards the right. Stenothermic species tend to have a more peaked curve indicating only narrow and typically low temperature ranges over which optimal growth is achieved. Wide-ranging eurythermic species (e.g., D, E, and H) probably exhibit the greatest possibilities for adapting rapidly to shifting thermal regimes driven by climate change.
Fig. 8.18. Growth rates of fish species at varying temperatures determined from laboratory studies. Stenothermic northern species (e.g.,A, B, C, and F) are grouped towards the lower temperatures on the left, whereas mesothermic southern species (e.g., G, I, and J) are grouped towards the right. Stenothermic species tend to have a more peaked curve indicating only narrow and typically low temperature ranges over which optimal growth is achieved. Wide-ranging eurythermic species (e.g., D, E, and H) probably exhibit the greatest possibilities for adapting rapidly to shifting thermal regimes driven by climate change. Thermal preferenda presumably optimize all internal physiological processes (i.e., benefits outweigh costs) in individual fish associated with digestion, growth, muscle (hence swimming) efficiency, gas exchange across gills, cellular respiration, reproduction, and so on. The relationship of temperature to such processes is perhaps most easily seen with respect to growth (e.g., increase in size or weight over time; Fig. 8.18). In addition to exhibiting higher growth rates at lower temperatures, arctic fish species also exhibit narrower ranges of temperature preference and tolerance (i.e., stenothermic; Fig. 8.18), which has profound effects on productivity. Stenothermic tolerances also imply that the species may have little capacity to accommodate thermal impacts of climate change. Conversely, species exhibiting eurythermic or wide thermal tolerances or responses are likely to have a much wider capacity to accommodate climate changes.
Population-level influences of thermal regimes are also apparent. Effects on individuals, such as temperature effects on mortality, feeding, parasitism, and predation, are integrated into consequences for fish populations through the various processes that connect fish populations to their ecosystems (Fig. 8.19). As noted previously, environmental parameters such as temperature may affect various life stages differently and thus can be modeled separately, but it is important to remember that the ultimate effects of all these influences are integrated throughout the fish population of interest. Similarly, environmental changes also have specific effects on other organisms relevant to fish, such as predators, parasites, and food organisms. Therefore, a single environmental parameter may exert both indirect and direct effects at many levels that influence the fish population, but the actual effect of this may be indiscernible from the effects of other natural and anthropogenic influences. Figure 8.19 provides examples of linkages between environmental parameters that affect key processes at the fish population level. Migratory aspects of life history are not shown in the figure, but will also (especially in anadromous fish) be significantly affected by abiotic processes.
Salinity will also be a factor for sea-run phases of adult life history. Climate change and increased variability in climate parameters will drive changes in aquatic abiotic parameters. Such changes will affect the fish directly as well as indirectly via impacts on their prey, predators, and parasites. This cascade of effects, and synergies and antagonisms among effects, greatly complicates the projection of climate change impacts on valued northern fish populations. In addition, other parameters not shown in Fig. 8.19, such as groundwater inflows to spawning beds, will affect the survival of various life-history stages. The ultimate effects of all these interacting factors will in turn affect sustainability of the populations and human uses in a fishery context.
Temperature effects on individual fish and fish populations are perhaps the most easily understood ones, however, other climate parameters such as precipitation (Precipitation and evapotranspiration in the Arctic) (amount and type) will directly affect particular aquatic environmental parameters such as productivity and flow regimes (amounts and timing). For example, flattening hydrographs and shifts in water sources (Sections 8.4.2 and 8.4.3 (Information required to project responses of arctic fish)) are very likely to alter the availability of arctic rivers as migratory routes for anadromous fish. Increased and earlier vernal flows are very likely to enhance fish survival during out-migration and lengthen the potential summer feeding period at sea (both positive effects at the levels of the individual fish and the population). However, autumnal flows are required in many smaller rivers to provide access to returning fish[13]; reduction in amounts and shifts in timing of these flows are very likely to have negative effects. Svenning and Gullestad[14] examined environmental influences on migrations of Arctic char, particularly local temperature effects on flow regimes and the consequences for fish population abundance and structure.
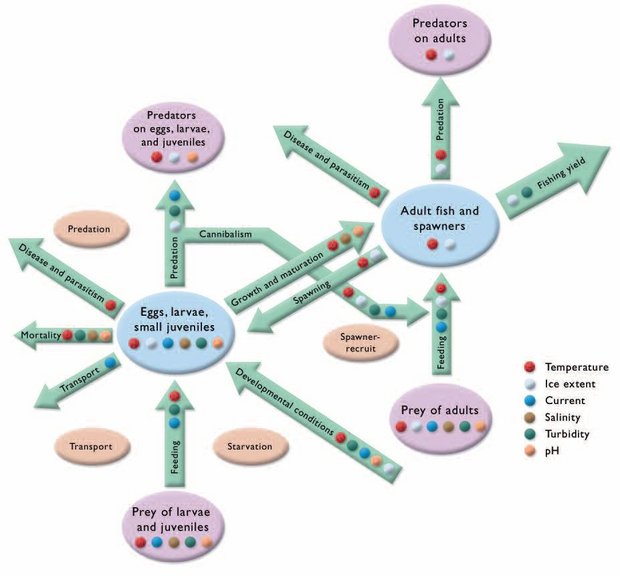 Fig. 8.19. The major biotic processes affecting the dynamics of a freshwater or anadromous arctic fish population and some of the aquatic abiotic environmental parameters that affect these processes. The tan ovals refer to the four major processes controlling fish production; these will shift as a result of climate change effects on the various components of the ecosystem[2].
Fig. 8.19. The major biotic processes affecting the dynamics of a freshwater or anadromous arctic fish population and some of the aquatic abiotic environmental parameters that affect these processes. The tan ovals refer to the four major processes controlling fish production; these will shift as a result of climate change effects on the various components of the ecosystem[2]. Additional secondary environmental factors that may change in response to direct changes in basic climate parameters will also have important effects on aquatic biota. These include the nature and duration of freeze-up, ice types, ice-cover periods, and breakup, and the nature and penetration of incident radiation into aquatic systems. Similarly, terrestrial impacts of climate change may influence aquatic habitat and indirectly affect its biota (e.g., permafrost (Permafrost in the Arctic) alteration and runoff (Freshwater discharge in the Arctic) influences on sediment loads, pH and related water chemistry, etc.). Another potential class of indirect effects of climate change includes those affecting the behavior of aquatic biota. For example, fish use thermal regimes and spatiotemporal shifts in these regimes, at least in part, as behavioral cues or thresholds to trigger critical life history functions. Water mass boundaries defined by temperature act as barriers to movement and may define feeding areas[15]. Final gametic maturation in autumn-spawning species is probably triggered by decreasing water temperatures and perhaps also photoperiod in arctic whitefishes. There is anecdotal evidence that decreased sediment loads resulting from freezing of riverbanks trigger final upstream movements by broad whitefish from holding areas to spawning sites[16], an adaptation to ensure eggs are not smothered. Water temperature integrated over time (e.g., as degree-days) affects the rate of egg development. Thus, aquatic thermal regimes affect ectotherms such as fish in two basic ways: by influencing physiology and as cues for behavioral changes. Although typically less understood, similar effects probably result from other physical (e.g., currents, flows, turbidity, ice dynamics) and chemical (e.g., pH, oxygen) parameters in the aquatic habitat[17]. Climate-induced alteration of these habitat characteristics is very likely to significantly affect arctic fish populations, although substantive research is required to quantify such effects.
Freshwater and diadromous fishes of the Arctic exhibit high diversity in the way that climate parameters affect their distributions, physiology, and ecology. These factors, together with the more complex indirect effects that climate may have upon their habitats, implies a wide range of possible responses to climate change. Other than logical extrapolations, most responses to climate change are impossible to quantify due to the absence of basic biological information for most arctic fish species and the incomplete understanding of the overall associations of ecological processes with present-day climate parameters.
Ecosystems, habitat, and fish: climate change in the context of multiple stressors (8.5.1.2)
Aquatic ecosystems are highly structured and complex entities consisting of both abiotic and biotic elements, and functional relationships within and between those elements. Similarly, individual components of ecosystems, such as a single fish species, exhibit a unique but complex structure. From the perspective of an individual or even a population, climate parameters and change in them may be experienced as either a stressor (i.e., that which perturbs homeostatic systems[18]), or as a promoter (i.e., that which promotes homeostasis). Stressors and promoters directly and indirectly influence underlying physiological processes and their outcomes at both the level of the individual and that of the population. Points of action within individuals range from the molecular to the organismal level; those affecting whole individuals overlap with effects on populations and communities[19]. Events occurring at other levels of the hierarchy also influence the various organizational levels. Levels within the individual tend to have short-term responses and limited overall ecological relevance, whereas those at the population/community/ecosystem level tend to be longer-term responses with higher ecological relevance[20]. Given the structured complexity inherent in a fish population, the effect of any particular stressor or promoter can be manifested at many levels simultaneously; can interact with others in an additive or cumulative fashion; and can typically be observed (if at all) in wild populations only at the more general population or community level.
For example, the effect of a temperature change may induce a short-term physiological response in an individual (e.g., processes occurring outside of the zone of optimal enzyme performance), a medium-term acclimatization response such as expression of new enzyme alleles optimal for the new temperature, and a somewhat longer-term response of changed biological condition. Concomitant expressions at the population level might be a shift in age structure, lower overall abundance, and ultimately local extirpation or adaptation to new conditions. Another important point is that at least locally, the impacts of climate change on fish populations will be but one of several stressors. Other stressors affecting arctic fish both now and in the future include exploitation, local habitat change due to industrial development or river regulation, contaminant loadings, and changes in incident UV radiation (Effects of changes in climate and UV radiation levels on structure of arctic ecosystems in the short and long term) levels. These stressors will result in effects similar to those described for climate change at individual and population levels. However, all these stressors will also interact additively and multiplicatively on individual fish and fish populations; hence, the effects are likely to be cumulative[21]. Perhaps the greatest future challenge associated with climate change will be to effectively recognize and manage in an integrated fashion all potential and realized impacts on arctic fish populations to ensure their conservation and sustainability.
Effects of climate-induced changes on physical habitat (8.5.1.3)
Physical changes in aquatic habitats will very probably affect arctic fishes as climate changes in the north. This section provides some examples to illustrate the linkages and various potential effects on biota, but the underlying absence of data precludes quantification of causal linkages in most cases. Rectifying these and similar knowledge gaps is a major future challenge.
Groundwater and fish
Groundwater flows sustain fish habitat and are extremely important during periods of low flow in many arctic rivers[22] and perhaps some lakes (Sections 8.2 and 8.4 (Information required to project responses of arctic fish)). For stream-dwelling salmonids, inflows along stream bottoms clear fine-grained sediments from spawning areas, supply thermally regulated and oxygenated water to developing eggs and larval fish, and in many cases provide physical living space for juvenile and adult fish. In highly channeled shallow arctic rivers that characterize many areas of the North American Arctic and Chukotka, groundwater inputs are critical to fish migrations and stranding prevention[23]. In winter, many Alaskan and western Canadian North Slope rivers cease flowing and freeze to the bottom over large stretches, and groundwater provides refugia that support entire populations of Arctic grayling and Dolly Varden as well as any co-occurring species[24]. Overwintering mortality, especially of adults weakened from spawning activities, is suspected as a primary regulator of the populations of Dolly Varden in this area and a major factor in such mortality is the quality and amount of winter habitat maintained by groundwater. Possible increases in groundwater flows resulting from climate change are likely to positively affect overwinter survival, especially if coupled with shorter duration and thinner ice cover. However, increased nutrient loadings in groundwater will possibly have more complex impacts (e.g., increases in in-stream primary and secondary productivity are likely to promote growth and survival of larval fish, but increases in winter oxygen demand associated with vegetation decomposition will possibly decrease overwinter survival of larger fish). How these various effects will balance in specific situations to result in an overall net effect on particular fish populations is unknown.
In summer, ground and surface water inflows ameliorate summer [[temperature]s] and provide thermal refugia, especially along southern distributional margins[25]. This is probably especially relevant for fish belonging to the arctic and coldwater thermal guilds. However, even the small increases in water temperatures (2 to 4°C) that are likely to result from climate change (e.g., warmer surface flows) will possibly preclude some species from specific aquatic habitats (e.g., temperature in higher-elevation cold-water stream reaches determines habitat occupancy of bull trout – Salvelinus confluentus[26]). Increased ambient conditions above physiological thermal optima are very likely to further stress populations and, combined with other possible effects such as competition from colonizing southern taxa, such impacts are likely to exacerbate range contractions for arctic species.
Ice and fish
The influence of ice on arctic fish and fish habitat is significant, especially in smaller lotic systems important to salmonids[27]. Effects include possible physical damage (e.g., from frazil ice), limitation of access to habitat (e.g., decreasing water volumes in winter due to ice growth), and annual recharge of habitat structure during dynamic breakup (e.g., cleansing of interstitial spaces in gravel). Shifts in the timing and duration of ice-related events are very likely to affect the survival and success of fish, with some effects being advantageous and others disadvantageous. In the north, these effects will be superimposed upon a poorly known but complex biological and environmental situation. Limited knowledge precludes accurate forecasting of many of these potential effects, and novel approaches are required to redress this[28].
Decoupling of environmental cues due to differential effects of climate change
A speculative issue, which may present surprises and unanticipated effects, is the potential for decoupling of various types of environmental drivers due to climate change affecting some differentially. Fish and other organisms use progressive and/or cusp-like changes in environmental parameters as cues to trigger key life-history functions such as migration, reproduction, and development. For example, although quantitative linkages are lacking, change in photoperiod (e.g., declining light period) is probably coupled with declining water [[temperature]s] in the autumn and together these trigger final gonadal maturation and reproductive activities in many northern fishes (especially salmonids). Environmental cues that drive major life-history events are especially critical for migratory species, and in the Arctic, particularly for anadromous species. This coupling is probably especially strong in the north where both parameters change rapidly on a seasonal basis. Although not explored to date in the context of climate change, as seasonal photoperiod shifts remain unchanged but coincident cues such as declining temperatures occur later in the autumn, such decoupling will possibly have profound impacts on population processes. The initial impact of such decoupling may be quite subtle (e.g., lowered fecundity, fertilization success, and/or egg survival in the previous example), not readily discernible, and almost certainly not directly attributable to climate change. However, a critical threshold is likely to be reached when impacts become significant (e.g., total reproductive failure in one year resulting in a failed year class, ultimately leading to population extirpation if it occurs over successive years approaching the generation time of the population). Investigation of coupling between cues, their influence upon population processes in fish and other aquatic organisms, and their potential for decoupling due to climate change in the Arctic should be a priority.
These are but a few examples of likely influences of physical habitat on fish populations and the potential effects of climate-induced change on them that will have cascading effects on the integrity, sustainability, and future productivity of northern fishes. These serve to illustrate the general lack of knowledge that exists regarding associations between physical habitat and biology in northern aquatic biota, and thus how the impacts of climate change impacts will manifest. Redress of this knowledge gap is required on a community- and/or species-specific basis to account for local and historical influences and filters, which greatly affect the present-day structure and function of these aquatic ecosystems[29].
Issues at the level of fish populations (8.5.1.4)
As implied previously, projecting climate change impacts at the population level for most species is complex and fraught with uncertainty, especially for arctic species for which there is a dearth of fundamental biological information. A variety of approaches to address this problem are available (Section 8.5.2 (Information required to project responses of arctic fish)) and most have been applied in one way or another to develop some understanding of climate change impacts on northern fish populations.
In North America, much of the research focus on climate change effects on freshwater fish populations and communities has been in the south, for example, in the Great Lakes region and associated fisheries (Freshwater ecosystems and fisheries in the Arctic)[30]. In that region, climate change is projected to result in effects similar to those projected for the Arctic (e.g., significant reductions in the duration and extent of ice cover, an earlier seasonal disappearance of the 4°C depth isotherm, measurable declines in DO, and slight hypolimnetic anoxia in shallower basins[31]). Loss of suitable cool-water habitat associated with lake warming is also projected, which will very probably differentially affect species within lacustrine fish communities (e.g., promote growth and survival in lake whitefish but negatively affect these in lake trout[32]). Preliminary consideration of northern areas has occurred for European systems[33]. Relatively less attention has been paid to the possible effects of climate change on resident fish communities in other ecosystems, particularly those in the Arctic. With respect to freshwater fish populations, the IPCC[34] concluded that fish populations in [[stream]s] and rivers on the margins of their geographic distributions (e.g., arctic and subarctic species) will be the first to respond to the effects of climate change because these systems have a high rate of heat transfer from the air. Some of these effects include:
- Nutrient level and mean summer discharge explained 56% of the variation in adult Arctic grayling growth over a 12-year period in two Alaskan rivers[35]. Summer temperature added to these variables explained 66% of variation in young-of-the-year growth. Correlation with discharge was positive for adults and negative for young, thus grayling life history appears able to respond to variability in the arctic environment by balancing adult growth with year-class strength. How this balance will shift under climate change is uncertain at present.
- Temperature effects on growth appear to be greatest at the extremes of the geographic range of the species[36], and local effects will be species-specific[37]. Generally, young-of-the-year fish appear to grow better in warmer summers and reach relatively larger sizes, predisposing them to higher overwinter survival, which determines year-class strength and population abundance[38]; potentially a positive result of climate change assuming food is not a limiting factor.
- Northern lake cisco (Coregonus artedi) populations along the coast of Hudson Bay exhibited reduced growth and later maturity due to lower temperatures and shorter growing seasons[39]. Individual fecundity did not change, but the most northerly populations skipped reproduction more frequently (hence overall population productivity was lower). This latitudinal gradient represents responses to temperature stresses whereby further trade-offs in energy allocation between reproduction and growth currently are not possible[40]; a common circumstance for most arctic fish populations and one that will probably be ameliorated under scenarios of increased temperature, potentially resulting in increased population abundances.
- Counter-gradient variation[41], whereby genetic influences on growth in species such as brown trout (Salmo trutta) vary inversely with mean annual water temperatures[42], suggest that trout in the coldest rivers are specifically adapted to low temperatures and short growing seasons. Thus, increased temperatures are likely to negatively affect growth rates, age/size structure, and abundances of northern populations.
Chapter 8: Freshwater Ecosystems and Fisheries
8.1. Introduction (Information required to project responses of arctic fish)
8.2. Freshwater ecosystems in the Arctic
8.3. Historical changes in freshwater ecosystems
8.4. Climate change effects
8.4.1. Broad-scale effects on freshwater systems
8.4.2. Effects on hydro-ecology of contributing basins
8.4.3. Effects on general hydro-ecology
8.4.4. Changes in aquatic biota and ecosystem structure and function
8.5. Climate change effects on arctic fish, fisheries, and aquatic wildlife
8.5.1. Information required to project responses of arctic fish
8.5.2. Approaches to projecting climate change effects on arctic fish populations
8.5.3. Climate change effects on arctic freshwater fish populations
8.5.4. Effects of climate change on arctic anadromous fish
8.5.5. Impacts on arctic freshwater and anadromous fisheries
8.5.6. Impacts on aquatic birds and mammals
8.6. Ultraviolet radiation effects on freshwater ecosystems
8.7. Global change and contaminants
8.8. Key findings, science gaps, and recommendations
References
Citation
Committee, I. (2012). Information required to project responses of arctic fish. Retrieved from http://editors.eol.org/eoearth/wiki/Information_required_to_project_responses_of_arctic_fish- ↑ Dahm, C.N. and M.C. Molles Jr., 1992. Streams in semi-arid regions as sensitive indicators of global climate change. In: P. Firth and S.G. Fisher (eds.). Troubled Waters of the Greenhouse Earth, pp. 250–260. Springer-Verlag.
- ↑ Beitinger, T.L. and L.C. Fitzpatrick, 1979. Physiological and ecological correlates of preferred temperature in fish. American Zoologist, 19:319–329.
- ↑ Coutant, C.C., 1987. Thermal preference: when does an asset become a liability? Environmental Biology of Fishes, 18:161–172.
- ↑ Craig, P.C., 1989. An introduction to anadromous fishes in the Alaskan Arctic. In: D.W. Norton (ed.). Research Advances on Anadromous Fish in Arctic Alaska and Canada. Biological Papers of the University of Alaska, 24:27–54.
- ↑ Magnuson, J.J., L.B. Crowder and P.A. Medvick, 1979. Temperature as an ecological resource. American Zoologist, 19:331–343.
- ↑ Reist, J.D., 1994. An overview of the possible effects of climate change on northern freshwater and anadromous fishes. In: S.J. Cohen (ed.). Mackenzie Basin Impact Study (MBIS), Interim Report 2, pp. 377–385. Environment Canada, Ottawa.
- ↑ Berra,T.M., 2001. Freshwater Fish Distribution. Academic Press, 604pp.
- ↑ Schlesinger, D.A. and H.A. Regier, 1983. Relationship between environmental temperature and yields of subarctic and temperate zone fish species. Canadian Journal of Fisheries and Aquatic Sciences, 40:1829–1837.
- ↑ Sibley, T.H. and R.M. Strickland, 1985. Fisheries: some relationships to climate change and marine environmental factors. In: M.R. White (ed.). Characterization of Information Requirements for Studies of CO2 Effects: Water, Resources, Agriculture, Fisheries, Forests and Human Health, pp. 95–143. DOE/ER-0236. United States Department of Energy, Washington, D.C.
- ↑ Schlesinger, D.A. and H.A. Regier, 1983. Relationship between environmental temperature and yields of subarctic and temperate zone fish species. Canadian Journal of Fisheries and Aquatic Sciences, 40:1829–1837.
- ↑ Shuter, B.J. and J.R. Post, 1990. Climate, population viability, and the zoogeography of temperate fishes. Transactions of the American Fisheries Society, 119:314–336.
- ↑ Shuter, B.J. and J.R. Post, 1990. Climate, population viability, and the zoogeography of temperate fishes. Transactions of the American Fisheries Society, 119:314–336.
- ↑ Jonsson, N., 1991. Influence of water flow, water temperature and light on fish migration in rivers. Nordic Journal of Freshwater Research, 66:20–35.
- ↑ Svenning, M.-A. and N. Gullestad, 2002. Adaptations to stochastic environmental variations: the effects of seasonal temperatures on the migratory window of Svalbard Arctic charr. Environmental Biology of Fishes, 64:165–174.
- ↑ Coutant, C.C., 1987. Thermal preference: when does an asset become a liability? Environmental Biology of Fishes, 18:161–172.
- ↑ Reist, J.D. and K. Chang-Kue, 1997. The life history and habitat usage of broad whitefish in the lower Mackenzie River basin. In: R.F. Tallman and J.D. Reist (eds.). The Proceedings of the Broad Whitefish Workshop: The Biology, Traditional Knowledge and Scientific Management of Broad Whitefish (Coregonus nasus (Pallas)) in the Lower Mackenzie River, pp. 63–84. Canadian Technical Report of Fisheries and Aquatic Sciences 2193.
- ↑ Sibley, T.H. and R.M. Strickland, 1985. Fisheries: some relationships to climate change and marine environmental factors. In: M.R. White (ed.). Characterization of Information Requirements for Studies of CO2 Effects: Water, Resources, Agriculture, Fisheries, Forests and Human Health, pp. 95–143. DOE/ER-0236. United States Department of Energy, Washington, D.C.
- ↑ Adams, S.M., 1990. Status and use of biological indicators for evaluating the effects of stress on fish. In: S.M. Adams (ed.). Biological Indicators of Stress in Fish. American Fisheries Society Symposium, 8:1–8.
- ↑ Adams, S.M., 1990. Status and use of biological indicators for evaluating the effects of stress on fish. In: S.M. Adams (ed.). Biological Indicators of Stress in Fish. American Fisheries Society Symposium, 8:1–8.
- ↑ Adams, S.M., 1990. Status and use of biological indicators for evaluating the effects of stress on fish. In: S.M. Adams (ed.). Biological Indicators of Stress in Fish. American Fisheries Society Symposium, 8:1–8.
- ↑ Reist, J.D., 1997b. Potential cumulative effects of human activities on broad whitefish populations in the lower Mackenzie River basin. In: R.F. Tallman and J.D. Reist (eds.). The Proceedings of the Broad Whitefish Workshop: The Biology, Traditional Knowledge and Scientific Management of Broad Whitefish (Coregonus nasus (Pallas)) in the Lower Mackenzie River, pp. 179–197. Canadian Technical Report of Fisheries and Aquatic Sciences 2193.
- ↑ Power, G., R.S. Brown and J.G. Imhof, 1999. Groundwater and fish – insights from northern North America. Hydrological Processes, 13:401–422.
- ↑ Power, G., R.S. Brown and J.G. Imhof, 1999. Groundwater and fish – insights from northern North America. Hydrological Processes, 13:401–422.
- ↑ Craig, P.C., 1989. An introduction to anadromous fishes in the Alaskan Arctic. In: D.W. Norton (ed.). Research Advances on Anadromous Fish in Arctic Alaska and Canada. Biological Papers of the University of Alaska, 24:27–54.
- ↑ Power, G., R.S. Brown and J.G. Imhof, 1999. Groundwater and fish – insights from northern North America. Hydrological Processes, 13:401–422.
- ↑ Paul, A.J. and J.R. Post, 2001. Spatial distribution of native and nonnative salmonids in streams of the eastern slopes of the Canadian Rocky Mountains. Transactions of the American Fisheries Society, 130:417–430.
- ↑ Craig, P.C., 1989. An introduction to anadromous fishes in the Alaskan Arctic. In: D.W. Norton (ed.). Research Advances on Anadromous Fish in Arctic Alaska and Canada. Biological Papers of the University of Alaska, 24:27–54.–Cunjak, R.A., T.D. Prowse and D.L. Parrish, 1998. Atlantic salmon (Salmo salar) in winter: the season of parr discontent? Canadian Journal of Fisheries and Aquatic Sciences, 55(S1):161–180.–Power, G., R.S. Brown and J.G. Imhof, 1999. Groundwater and fish – insights from northern North America. Hydrological Processes, 13:401–422.–Prowse, T.D., 2001a. River-ice ecology. I: hydrology, geomorphic, and water-quality aspects. Journal of Cold Regions Engineering, 15:1–16.–Prowse, T.D., 2001b. River-ice ecology. II: biological aspects. Journal of Cold Regions Engineering, 15:17–33.
- ↑ Cunjak, R.A., T.D. Prowse and D.L. Parrish, 1998. Atlantic salmon (Salmo salar) in winter: the season of parr discontent? Canadian Journal of Fisheries and Aquatic Sciences, 55(S1):161–180.
- ↑ Tonn, W.M., 1990. Climate change and fish communities: a conceptual framework. Transactions of the American Fisheries Society, 119:337–352.
- ↑ Assel, R.A., 1991. Implications of CO2 global warming on Great Lakes ice cover. Climatic Change, 18:377–395.–Hill, D.K. and J.J. Magnuson, 1990. Potential effects of global climate warming on the growth and prey consumption of Great Lakes fish. Transactions of the American Fisheries Society, 119:265–275.–Magnuson, J.J., J.D. Meisner and D.K. Hill, 1990. Potential changes in the thermal habitat of Great Lakes fish after global climate warming. Transactions of the American Fisheries Society, 119:254–264.–Meisner, J.D., J.L. Goodier, H.A. Regier, B.J. Shuter and W.J. Christie, 1987. An assessment of the effects of climate warming on Great Lakes basin fishes. Journal of Great Lakes Research, 13:340–352.–Minns, C.K. and J.E. Moore, 1992. Predicting the impact of climate change on the spatial pattern of freshwater fish yield capability in eastern Canadian lakes. Climatic Change, 22:327–346.–Regier, H.A., J.J. Magnuson and C.C. Coutant, 1990. Introduction to proceedings: symposium on effects of climate change on fish. Transactions of the American Fisheries Society, 119:173–175.–Regier, H.A., P. Lin, K.K. Ing and G.A. Wichert, 1996. Likely responses to climate change of fish associations in the Laurentian Great Lakes Basin: concepts, methods and findings. Boreal Environment Research, 1:1–15.–Shuter, B.J. and J.R. Post, 1990. Climate, population viability, and the zoogeography of temperate fishes. Transactions of the American Fisheries Society, 119:314–336.–Smith, J.B., 1991. Potential impacts of climate change on the Great Lakes. Bulletin of the American Meteorological Society, 72:21–28.
- ↑ Blumberg, A.F. and D.M. Di Toro, 1990. Effects of climate warming on dissolved oxygen concentrations in Lake Erie. Transactions of the American Fisheries Society, 119:210–223.–Schertzer, W.M. and A.M. Sawchuk, 1990. Thermal structure of the lower Great Lakes in a warm year: implications for the occurrence of hypolimnion anoxia. Transactions of the American Fisheries Society, 119:195–209.
- ↑ Magnuson, J.J., J.D. Meisner and D.K. Hill, 1990. Potential changes in the thermal habitat of Great Lakes fish after global climate warming. Transactions of the American Fisheries Society, 119:254–264.
- ↑ Lehtonen, H., 1996. Potential effects of global warming on northern European freshwater fish and fisheries. Fisheries Management and Ecology, 3:59–71.
- ↑ Arnell, N., B. Bates, H. Lang, J.J. Magnuson, P. Mulholland, S. Fisher, C. Liu, D. McKnight, O. Starosolszky and M.Taylor, 1996. Hydrology and freshwater ecology. In: R.T. Watson, M. Zinyowera and R.H. Moss (eds.). Climate Change 1995: Impacts, Adaptations and Mitigation of Climate Change: Scientific-Technical Analyses. Contribution of Working Group II to the Second Assessment Report of the Intergovernmental Panel on Climate Change, pp. 325–363. Cambridge University Press.
- ↑ Deegan, L.A., H.E. Golden, C.J. Harvey and B.J. Peterson, 1999. Influence of environmental variability on the growth of age-0 and adult Arctic grayling. Transactions of the American Fisheries Society, 128:1163–1175.
- ↑ Power, M. and M.R. van den Heuvel, 1999. Age-0 yellow perch growth and its relationship to temperature.Transactions of the American Fisheries Society, 128:687–700.
- ↑ King, J.R., B.J. Shuter and A.P. Zimmerman, 1999. Empirical links between thermal habitat, fish growth, and climate change. Transactions of the American Fisheries Society, 128:656–665.
- ↑ Shuter, B.J., J.A. MacLean, F.E.J. Fry and H.A. Regier, 1980. Stochastic simulation of temperature effects on first year survival of smallmouth bass.Transactions of the American Fisheries Society, 109:1–34.
- ↑ Morin, R., J.J. Dodson and G. Power, 1982. Life history variations of anadromous cisco (Coregonus artedii), lake whitefish (C. clupeaformis), and round whitefish (Prosopium cylindraceum) populations of eastern James-Hudson Bay. Canadian Journal of Fisheries and Aquatic Sciences, 39:958–967.
- ↑ Morin, R., J.J. Dodson and G. Power, 1982. Life history variations of anadromous cisco (Coregonus artedii), lake whitefish (C. clupeaformis), and round whitefish (Prosopium cylindraceum) populations of eastern James-Hudson Bay. Canadian Journal of Fisheries and Aquatic Sciences, 39:958–967.
- ↑ Levins, R., 1969. Thermal acclimation and heat resistance in Drosophila species.The American Naturalist, 103:483–499.
- ↑ Jensen, A.J., T. Forseth and B.O. Johnsen, 2000. Latitudinal variation in growth of young brown trout Salmo trutta. Journal of Animal Ecology, 69:1010–1020.
- ↑ Reist, J.D., 1994. An overview of the possible effects of climate change on northern freshwater and anadromous fishes. In: S.J. Cohen (ed.). Mackenzie Basin Impact Study (MBIS), Interim Report 2, pp. 377–385. Environment Canada, Ottawa.