Technology and the materials cycle
| Topics: |
Geology (main)
|
Technology and the materials cycle interact in a manner that disrupts the cycling of minerals and metal cycles in the natural environment.
Technology is the human application of knowledge to practical purpose. Through an ongoing process of discovery and knowledge building, humans apply technological knowledge to meet the needs of individuals, industries and society. Technology changes in response to increased knowledge and the shifting priorities among economic, political, or societal factors. Technology is built upon accumulated knowledge about how best to organize capital, energy, information, labor and material resources.
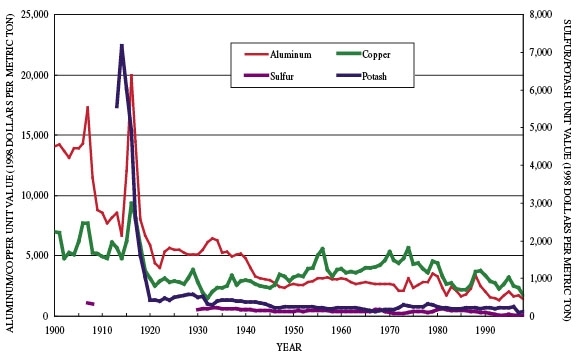
Figure 1: Unit values for selected minerals, 1900 to 1998 (Buckingham and Ober, 2002; Buckingham and Plunkert, 2002; Buckingham and Searls, 2002; Porter and Edelstein, 2002). The unit value (1998 dollars per metric ton) is defined as 1 metric ton of apparent consumption estimated from the “Annual Average Primary Price” in U.S. dollars as reported by the U.S. Geological Survey (1999), divided by the Consumer Price Index with a base year of 1998.
Several common themes emerge from the history of technological changes in mineral-commodity industries. Despite their long histories, natural resource industries have managed to maintain a high level of technological sophistication through continual incremental innovation and restructuring. These industries have made use of technological innovations from within and outside their industry. Innovations generally are not generally revolutionary, rather they are evolutionary.
Greater understanding of geologic mineral-deposit models has aided the discovery of numerous resource types. Technology has been developed to extract and process many of these diverse resources, and mineral supply has expanded as a result.
| Commodity | Percentage increase in production |
Percentage decrease (in 1998 dollar unit value)1 |
| Aluminum | 31,000 | 84 |
| Copper | 800 | 75 |
| Potash | 148,000 | 87 |
| Sulfur | 13,000 | 85 |
| 1. The real value (1998 dollars per metric ton) is defined as 1 metric ton of apparent consumption as reported by the U.S. Geological Survey (1999) divided by the Consumer Price Index with a base year of 1998. (Source: Buckingham and Ober, 2002; Buckingham and Plunkert, 2002; Buckingham and Searls, 2002; Porter and Edelstein, 2002) | ||
Most natural-resource producers have accommodated increased concerns for environmental protection and worker health and safety and yet remain competitive. Data collected by the United States Geological Survey suggests that during the past 100 years, world production of nonfuel minerals has managed to meet increased demand at lower real prices. Figure 1 and table 1 show production/price (expressed in 1998 dollars) relationships during the 20th century for the four commodities discussed as case studies in Appendices 2 through 5.
Between 1900 and 1998, world primary plus secondary aluminum metal production increased by about 31,000 percent, but the aluminum metal price decreased in constant dollar terms by 84 percent. For the same period, world copper metal production increased by about 800 percent, but its price decreased by 75 percent. A similar trend is apparent for industrial minerals. Since 1900, world potash production increased by 148,000 percent, but its price decreased by 87 percent. Also, world sulfur production increased by 13,000 percent, but its price decreased by 85 percent. Advances in technology played a significant part in these trends.
Although circumstances or social pressures, such as >environmental regulation or localized resource shortages, may initiate technological change, innovations tend to generate additional change most often on an incremental level. When an industry is capital intensive (that is, has invested large amounts of money on facilities or equipment), its owners have an incentive to perpetuate the industry. They can do so by investing in technologies to reduce production costs (Technological change in the copper industry).
Technological innovations and their applications are difficult to predict. During the past 50 years, advances that used such materials as silicon in computer chips, gallium in semiconductors, and nickel, cadmium, and lithium in batteries and printed circuits have had enormous impact on the electronics industry (figure 2). In 1949, Popular Science wrote that the Electronic Numerical Integrator and Calculator, which was the most sophisticated computer of the day, required 18,000 vacuum tubes and weighed 30 metric tons (t). The magazine predicted that computers in the future might require only 1,000 tubes and weigh only 1.5 t. The computer power that was in those 30 t can now fit in a modern pocket calculator that can be widely purchased at a low price. These advances have resulted in the use of diverse materials, lower weight, improved durability, decreased energy consumption, and increased performance at markedly lower costs.
President Truman created the President’s Materials Policy (Paley) Commission in 1951 for the purpose of addressing concerns about domestic shortages of copper and other strategic materials during the Korean conflict and suggesting long-term solutions. The Commission issued the five-volume “Resources for Freedom” report in June 1952. The second volume, “The Outlook for Key Commodities,” included an analysis of copper supply. The findings were pessimistic about the Nation’s future copper supply from domestic. The Commission reported that U.S. reserves of copper ore, which contained at least 1 percent copper, were fixed in size and not likely to include lower grade ores. The copper industry, however, performed much better than expected. The average ore grade of copper extracted from U.S. mines in the early 1970s approached 0.6 percent. The average U.S. copper ore grade in 2000 approached 0.5 percent. The ability to mine these low-grade ores at a profit was generally achieved through technological improvements.
Productivity can serve, in part, as a measure of the impact of technology. Productivity is used as a measure of the efficiency of the production process. Implementation of the solvent extraction-electrowinning process (SX-EW), which requires a small workforce to recover large quantities of low-cost copper resources, is one major innovation that has helped sustain the copper industry since 1985. Other factors, such as increased capital investment, industry consolidation, and labor contract changes, contributed to improved productivity after 1985.
Contents
- 1 Application of Technology to the Materials Cycle
- 2 Conclusions Predictions of resource scarcity occur periodically. This study shows how the implementation of technology, which is the organization of energy, knowledge, labor, and materials, has historically addressed scarcity concerns. Although we know intuitively that resources are finite, the historical evidence suggests that resource availability has grown consistently and that real prices (by some measures) for materials during the past 100 years have trended downward or remained relatively steady. This has taken place in the face of declining ore grades, increasing demands by a population that is growing and has increasing lifestyle expectations, and mining resources that are increasingly difficult to access because of their depth, geologic complexity, remoteness, and (or) susceptibility to adverse political and social actions. Productivity improvements and an expanding diversity of resources from which materials can be profitably extracted and manufactured have come about through advances in technology. Technological developments are driven by the desire to supply material at the highest possible profit. (Technology and the materials cycle)
- 3 Further Reading
Application of Technology to the Materials Cycle
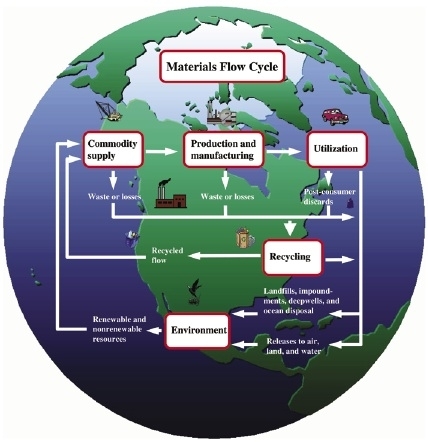
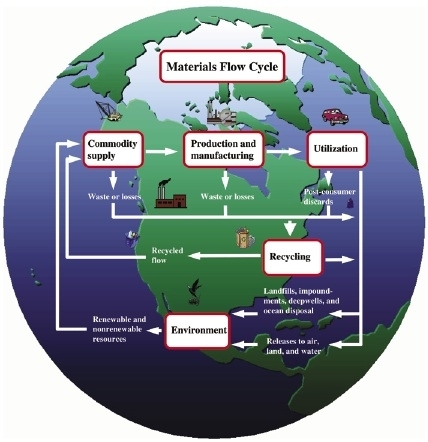
Figure 3: The materials flow cycle. Adapted from U.S. Geological Survey, 1998.
Technology improves the efficiency of the flow of minerals and materials to meet the needs of the consumer (figure 3). Technological improvements focus on developing methods to facilitate or improve the efficiency of exploration and development; extraction, processing, and fabrication; and product use and disposal. The technological methods used in each sector of these “nodes” of the materials cycle depend upon economics, environmental consequences, the relative political power of competing interests, and societal effects.
 Figure 4: Wastes deposited in landfills may become significant sources of materials in the future. (Source: A. MacIntire, DB Enterprises Inc., August 22, 2000)
Figure 4: Wastes deposited in landfills may become significant sources of materials in the future. (Source: A. MacIntire, DB Enterprises Inc., August 22, 2000)
Minerals exploration requires the ability to use techniques to locate economically recoverable resources. Mining technology allows the valuable resources to be extracted from the Earth. Processing technology provides a means of preferentially separating useful mineral constituents from undesirable minerals and materials by biological, chemical, and physical methods. The breaking of chemical and physical bonds (mineral processing) to extract useful substances often requires high-energy requirements that technological advances strive to minimize. Manufacturing technology creates combinations of materials to form desired products. Consumer products are then distributed to locations where their useful properties are needed; this process requires transportation technology. Some materials are lost to the environment during processing or transportation; technological advances can minimize these losses and their effects on the environment. As products become obsolete, they are recycled or disposed of in landfills where they are potential resources for future recovery (figure 4).
The following sections discuss the role of technology as it applies to the materials flow cycle for minerals. The cycle includes mineral exploration and extraction; processing and fabrication; use; and recycling, which includes remanufacturing and reuse. Figure 3 is a generalized flow diagram of the materials flow cycle.
Exploration: "Finding the Needle in the Haystack"
The history of mineral exploration shows the incremental and mutually reinforcing nature of technology. Throughout human history, technology has influenced where and how prospectors look for minerals by drawing upon mineral-deposit models that were developed from previous exploration experiences and mining activities, available technological tools, current knowledge of the earth sciences, and mining and processing capabilities. As knowledge grew and technology progressed, mineral-deposit models were gradually refined, new search areas were identified, and prospecting methods and equipment became more sophisticated. (Additional information can be found in History of mining exploration technology.)
The increasing sophistication of mineral-deposit models allowed reevaluation of known resource areas or application to other areas with similar geologic characteristics. The exploration histories of the Carlin Trend gold deposits in Nevada and the Olympic Dam copper/uranium deposits in Australia, which are discussed in History of mining exploration technology, are two examples that show how technological development in ore processing and geologic modeling led to the discovery of additional resources. Fine-grained gold from the Carlin Trend became an economically viable resource only after such technologies as cyanide leaching were developed to extract it.
Technological advances in processing ores encourage exploration for a greater variety of resource types. Lower grade porphyry copper ore bodies became more attractive for exploration with the advent of froth flotation to separate valuable minerals from waste material, large-scale open pit mining, Pierce-Smith converting, and a large-scale copper-refining process that uses oxygen as the reacting agent. Waste dumps used by U.S. copper producers became exploitable following the development of the SX-EW process, which allowed waste material from previous mining to be reprocessed for copper. Application of the Olympic Dam copper/uranium model to Europe and theUnited States has lead to the expansion of mineral supply by identifying unknown deposits. New deposit information, redefined deposit models, and (or) technological breakthroughs may make it possible for economic recovery of material that was considered to be noneconomic.
Major innovations that transformed exploration technology can borrow from breakthroughs made in other sciences and industries. Geologists applied or adapted some of the technological advances in geochemistry, geophysics, and oil and gas drilling to aid in mineral exploration. Computer and laboratory facilities provided the means to measure newly discovered resources with increasing accuracy. Long-range exploration and characterization efforts reduced environmental disturbance. By the end of the 20th century, exploration and mining used sophisticated models, such as those developed by the USGS and based on integrated science. The newer models rely heavily on computer modeling of grade and tonnage. Tools have been developed to detect mineral deposits under water and at great depths below the Earth’s surface. Remote sensing methods, such as satellite imagery, can provide a means of identifying areas with mineral-deposit potential without the need to visit remote sites or to disturb environmentally sensitive areas.
During the past 50 years, mineral exploration has located significant nonfuel mineral resources of economic interest several kilometers below the oceans’ surface. Extensive but thin deposits of manganese nodules and crusts that contain important amounts of cobalt, copper, and nickel have been located on the floor of most of the oceans of the world by using geochemical, geophysical, and remote sampling techniques. First discovered in the 1870s, manganese nodules were considered to be geologic curiosities. The potential of these deposits as sources of cobalt, copper, manganese, and nickel was not appreciated until the 1950s. Between 1958 and 1968, numerous companies began serious prospecting of the nodule fields to estimate their economic potential. By 1974, 100 years after the first samples were taken, exploration documented that the Clarion-Clipperton zone, which is one broad belt of seafloor between Hawaii and, was literally paved with nodules over an area of more than 3.5 million square kilometers. The area has been estimated to contain 7.5 billion metric tons of manganese, 340 million metric tons (Mt) of nickel, 265 Mt of copper, and 78 Mt of cobalt.
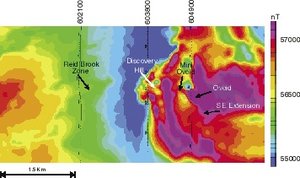
The application of geophysical methods to minerals exploration after World War II led to the discovery of many deposits and the extension of known ones. Airborne electromagnetic surveying (AEM) was developed in the late 1940s to differentiate highly conductive ore bodies from low-conductivity host rocks (figure 5). During the next 25 years, AEM was useful in the discovery of diamondiferous kimberlite pipes on the Canadian Shield, uranium deposits, and volcanogenic massive sulfides, as shown in figure 6. The frequency of discovery of these types of deposits increased significantly as exploration took place in a wide variety of geologic environments.
Gravity methods have been used since the 1950s to detect density contrasts between the dense mineral deposits and the less dense host rock. Although gravity anomalies often are difficult to discern and interpret, gravity methods have been used to investigate the exploration targets detected by AEM, geochemical, or magnetic surveys and can serve as a screening device that provides additional evidence to support continued exploration. Gravity methods have been successful in exploring for many types of deposits, which include natural gas, petroleum, porphyry [[copper]s], and sulfur.
Mineral exploration with magnetic methods, which detect contrasts between host rocks and magnetic mineralized zones, are most useful with iron ore deposit models, but they also aid in exploration for other deposits and can be used in advanced stages of exploration to define target ore zones better . The use of these geophysical tools as well as aerial photography and computer modeling has dramatically altered the scope of mineral exploration.
Extraction: “Separating the Needle from the Haystack”
Once a deposit has been located, technologies are required to determine if it is economically and technically viable to develop. Advances in blasting, such as the development of ammonium-nitrate fuel oil (ANFO) blasting agent, drilling, and equipment design and capacity have reduced costs and improved mining efficiency. The use of larger capacity equipment and increased mechanization has also improved productivity and reduced mining costs (figure 7). Mine planning and ore extraction have become more efficient and less costly because of computer technologies that enable realtime deposit modeling, sampling, and analysis of ores. The case studies in Appendices 2 through 5 describe how technology has been used to improve extraction and separation capabilities of aluminum, copper, potash, and sulfur ores, thereby expanding mineral supply.
As with many natural resource industries, technological advances in the copper industry consist of occasional major changes combined with continuous incremental improvements. Large-scale copper reduction became possible between 1905 and 1930 with the implementation of large-scale open pit mining, Pierce-Smith copper converters, and sulfide flotation. Pierce-Smith technology, which was developed in 1910, allowed copper to be enriched to between 95 and 97 percent at lower costs while consuming less energy than previous methods. The advent of flotation technology in Australia in the 1920s enabled the separation of copper from the unwanted material in copper-rich sulfides and separated different metal sulfides from each other, thus making copper available from polymetallic sulfide ore bodies. This technology led to byproduct recovery of what had been considered to be waste. Many ores in Mexico, for example, could now be extracted economically. The development of porphyry copper deposits in the Southwestern United States was made possible through these technological developments and improvements in open pit mining and materials-handling equipment. In the 10 years that followed the development of the first large porphyry copper mine (1906), output from mining these low-grade ore bodies amounted to 35 percent of U.S. copper production.
The history of the U.S. copper industry illustrates how technology contributed to the recovery of a declining industry confronted with trends of decreasing ore grades, expanding overseas competition and environmental regulation, and increasing ore complexity. Legislation that mandated higher ambient-air-quality standards with respect to sulfur dioxide (SO2) emissions (Emissions factors) led to the oxygen-flash-smelting process, which decreased energy requirements for copper smelting by 10 to 30 percent and, like other smelting processes, also produced SO2 that could be recovered and converted into sulfuric acid thereby lowering atmospheric emissions. This byproduct is used as the leaching agent in the SX-EW process, which allowed producers to recover copper from waste dumps, thus effectively extending mine life and rejuvenating the domestic copper mining industry. This process is widely used throughout Chile and the Southwestern United States and has been extended and combined with subsequent innovations so that even very low grade oxide ores now are economically recoverable.
Copper production predates aluminum production because of the absence of technology to separate aluminum from abundant aluminum-bearing clays or rocks. In the 1880s, development of the Bayer process to produce alumina from bauxite and the Hall-Héroult process to refine alumina into aluminum metal effectively allowed aluminum to be converted from a rare metal to a marketable commodity. The estimated (for indicative purposes only) 1887 unit value, in 1998 dollars, was about $300,000 per ton. In 1900, the estimated price was about $14,000 per ton, and by 1998 the price was about $1,400 per ton. Although the basic process has changed little since its inception, individual cell production capacity has increased to about 820 metric tons per year (t/yr) in 1995 from 15 to 20 t/yr in 1900. As a result of continuous improvements in cell design and process efficiency, cell efficiency increased to 92 to 95 percent in 1995 from 70 to 80 percent in 1900, and energy consumption decreased to about 13 kilowatt hours per kilogram (kWh/kg) of aluminum in 1995 from about 35 kWh/kg of aluminum in 1900.
Because aluminum production consumes large amounts of energy, early plants were located close to energy sources, which were mainly in Europe (coal) and the United States (Energy profile of the United States) (hydropower). As energy efficiencies were achieved after World War II and transportation costs increased, transportation factors also became important considerations in site selection. Integrated mining and processing facilities were developed in Saudi Arabia, South America, and Western Australia to take advantage of abundant bauxite resources and cheap local energy.
The United States, which has limited bauxite resources, relies on foreign sources of bauxite to supply the integrated domestic aluminum smelting and refining industry. Efficient materials handling and long-distance transportation systems have been developed to move large quantities of alumina and bauxite more cost effectively.
The potash case study shows how technology developed for one industry can be adapted to fit the needs of another (Technological change in the potash industry). Much of the world’s potash comes from salt deposits formed by the evaporation of ancient lakes, oceans, and seas. Deposits in Canada, Europe, and the United States were discovered in the first half of the 20th century during the search for petroleum. Within the past 100 years, diverse and very large potash resources have been discovered by using new technologies. These resources should be sufficient to supply the world’s potash needs well into the future. The deep deposits discovered in Canada were accessed by newly developed technologies that permitted shafts to penetrate rocks that contained water under high pressure. Continuous miners, which are highly mechanized pieces of equipment that are capable of producing up to 900 metric tons per hour from underground mines, were developed in the coal industry to improve productivity and safety and were adapted for use in potash mining (figure 8). Technology that is used to recover potash and sulfur through deep wells (solution mining) was adapted from the petroleum industry and is similar in principle to the solution mining techniques that were used by Chinese miners about 500 AD. Solar evaporation mining is used to recover potash from brines in the Middle East, the high deserts of Chile, and the Great Salt Lake in Utah. Flotation methods, which were originally developed by the copper industry, were adapted for use at potash operations in New Mexico during the 1930s and were quickly adopted worldwide.
The history of sulfur extraction and production technology also reflects continuous improvement of processes developed from other industries to meet changing materials use requirements and societal needs. The Frasch process, which produces elemental sulfur from underground deposits of native sulfur, was the first in situ mining technology used for sulfur production. This method, which has been used continuously since 1904, recovers sulfur from deposits that are located deep underground or underwater with little disturbance to the host rocks. The Claus process was first used in the 1950s to recover sulfur from the hydrogen sulfide produced during oil refining (figure 9). Sulfur in the form of hydrogen sulfide was found to be detrimental in the oil refining process because it is highly corrosive.
As energy demand grew between 1950 and 1975, sulfur production as a byproduct of oil refining also grew. The Clean Air Act of 1970 set standards for coal combustion for electricity generation, pyrometallurgical processing of sulfide ores, and sulfur recovery from oil refining, which stimulated the development of sulfur-removal technologies to comply with the new standards. Sulfur dioxide gas generated from oil refining and sulfide ore smelting is well-suited for the production of sulfuric acid. The petroleum and copper industries can use sulfuric acid effectively in other on-site processes. Since 1975, sulfur and sulfuric acid production from oil refining and sulfuric acid production during pyrometallurgical processing have come to dominate U.S. sulfur production. This is an example of achieving benefits from the conversion of a waste to a useful product.
Sulfur technologies were developed to meet industry challenges. Sulfur recovery technology has increased production and the number of production sources to the level that sulfur for the world economy is more than sufficient in the foreseeable future. At the same time, these technologies have allowed U.S. industries to comply with environmental regulations and provided other countries with technological models for environmental sustainability and industrial cooperation.
Development of the portland cement process revolutionized the cement industry during the 19th century. The portland process, in which a proportioned mixture of finely ground raw materials is converted to “clinker” by heating these materials until partial fusion takes place, allows a wider variety of natural raw materials to produce hydraulic cement with uniform strength and setting time; natural hydraulic cements, which set and harden underwater, are limited in nature and vary widely in quality and processing characteristics. Use of the rotary kiln, which was first introduced to process portland cement, led to development of alternative fuel sources, such as natural gas, petroleum, and powdered coal; increased cement production; and reduced labor costs. U.S. industry consumption of cement increased to 110 Mt in 2000 from 3.1 Mt in 1900. The U.S. construction industry uses this material in most commercial, public, and residential construction projects.
Current materials research on nickel illustrates how technology continues to be developed, even in times of abundant supply. Commercial application of several processes that use bacteria to concentrate metals contained in nickel ore (including cobalt, copper, and nickel) is very close to commercial implementation. By the year 2010, a process could come on-stream that will rely on a strain of bacteria that is usually associated with volcanic hot springs and is similar to those responsible for the formation of deep-sea metal deposits. The bacteria convert the sulfides to sulfates, which are a more easily treatable form. If proven successful, more nickel, as well as cobalt, copper, and other associated metals, could be recovered than with conventional processing methods because lower grade ores, previously mined materials, and more efficient extraction of nickel from traditional ores may be accomplished. This bacterial process also may have environmental advantages over established technologies because conventional smelting of nickel concentrate produces undesirable sulfur dioxide and dusts. Leach residue from this new method conforms to the U.S. Environmental Protection Agency’s (EPA) standards for stability. Moreover, capital costs are expected to be considerably lower than for conventional operations, and because the energy-intensive requirements and environmental costs associated with conventional smelting are substantially reduced, operating costs are said to be about 85 percent lower. This process and others that use bacteria have the potential to increase the availability of nickel and its related byproducts by enabling the mining of lower grade deposits and being “friendlier” to the environment.
Minerals have been explored for and recovered from ocean water for centuries. Offshore minerals, however, have been recovered from the ocean floor by using mechanized means for just more than a century (figure 10). For example, dredging for tin and titanium minerals, and, more recently, diamond has proven to be economically successful and allows recovery of these minerals from offshore sources. Although diamond recovery off the coast of Namibia has been occurring for some time; in 1996 mining in waters that approached 100 meters (m) in depth was initiated with the use of remote-controlled vehicles; the value of the diamond was approximately $2 million. Aggregate and sand for construction also have been mined offshore for many years.
Successful recovery of precious metals and artifacts from great depths and successful near-shore mining ventures have encouraged incremental technological advancements to recover materials from progressively deeper waters. Associated technological and environmental challenges include developing efficient mining techniques that are capable of raising material from great depths, processing it on ships, and disposing of waste in a manner that limits ecological damage.
The amount of infrastructure required to develop seabed resources is less than that for many land-based resources. Another economic advantage over most large-scale land mining is that most startup costs are much less; when mining is completed at one site, the operation can be moved easily to mine resources at another location. At least one study indicated that after a recovery period of as little as 6 months, mined offshore areas are biologically indistinguishable from unmined areas. As land-based sources become exhausted or unavailable, mining of urban [[coast]al areas] may increase.
Research in 2000 focused on the potential feasibility of recovering metals, primarily copper, lead, zinc, gold, and silver, deposited in ocean sediments near undersea hydrothermal vents. As many as 200 sites of inactive or previously active vents that deposited metals from underwater hot springs have been located; more discoveries undoubtedly will add to this number. Although few of these deposits have been closely examined and none has been fully evaluated for economic feasibility, grade, and size, some appear to have higher grades and tonnages than many of the onshore deposits currently being mined. Like manganese nodules and crusts, these undersea deposits are potentially huge resources. Many of the deposits have much higher grades and occur in shallower water than manganese nodules (2,000 m vs. 5,000 m), thereby presenting fewer economic and technological challenges.
Extraction of undersea resources from great depths requires technologies that can operate successfully in the harsh environments that are produced by darkness, extremely high [[pressure]s], and low temperatures. Space technologies, which include advanced materials, advanced power systems, intelligent sensors, multiband communications, remote sensing, and robotics, have potential applications in these environments. Numerous mining systems, such as suction and continuous bucket dredging and remote-controlled vehicles that collect and crush material before returning it to the surface for processing, are being tested.
Although mining mineral resources at great depths undersea is technologically possible, the high costs associated with mining and treating these ores, difficult environmental issues, and political and international legalities regarding ownership of minerals in international waters still are considered to be major obstacles that impede their development. Locating large high-grade deposits and granting exploration and mining permits within a country’s Exclusive Economic Zone (EEZ) may avoid some of the legal and political challenges. In 1997, an exploration license was granted for exploration and evaluation of a large high-grade deposit located within Papua New Guinea’s EEZ. Papua New Guinea recently granted mining licenses for tracts of seabed to a company that hoped to exploit underwater volcanic vents. On the basis of exploration results and the level of research on ocean mining technology, this resource has the potential to be developed in the next decade.
New technologies, however, are not always better. In some cases, newly developed technologies prove inferior to those that they were designed to replace or led to unintended consequences. For example, the U.S. Department of Energy conducted research into using thermonuclear devices as explosives for large-scale construction projects from the late 1950s to the early 1970s. Ideas proposed for testing included building sea-level canals (the Aleutians, Malaysia (Kra Isthmus), and Panama), building dams, dredging harbors on continental [[coast]s], recovering gas and oil from selected rocks, such as oil shale, and redirecting rivers.
In 1967, the U.S. Atomic Energy Commission exploded a 29-kiloton nuclear bomb underground in New Mexico. Project Gasbuggy, as it was designated, produced natural gas, which was radioactive and unusable. Project Rulison, which was another nuclear test conducted in 1969 in western Colorado, produced some gas and shock waves, which damaged building foundations, irrigation lines, local mines, and other structures, and resulted in concern by environmental groups and citizen backlash. Similar tests in the Soviet Union conducted between 1967 and 1978 left major areas contaminated with radionuclides and killed forests . Although this technology held the promise of being able to move massive amounts of material with minimal cost, it came with the unintended consequences of regional contamination and ecological alteration. Perhaps these problems could have been solved, but the projects were halted when nuclear test ban treaties were initiated during the 1980s.
Fabrication: “Threading the Needle”
Once ore is extracted and processed into a basic marketable commodity, such as aluminum or copper ingot, iron ore pellets, or potash crystals, it may need to be converted to a form that makes it readily usable by a manufacturer. Metals often are shaped into bars, rolls, or sheets. Industrial minerals frequently are formed into pastes, pellets, or powders. Blending materials, such as alloying metals, can change material characteristics and provide greater longevity, strength, and (or) versatility. As the needs of society change, technology develops new forms or redesigns existing ones to meet those needs.
The aluminum case study provides one example of how technological developments in fabrication significantly contributed to the growth of a mineral-resource industry (Technological change in the aluminum industry). Aluminum markets remained specialized until World War II when the low-weight, high-strength properties of aluminum made it the metal of choice for the structural components of aircraft. The technological improvements in casting and rolling that produced the large quantities of aluminum sheet necessary for the war effort and increased the adaptability and versatility of aluminum led to diversified and expanded postwar markets. Improvements in fabrication technology gradually allowed thinner sheets of aluminum to be produced and complex shapes to be formed and stamped. Aluminum was introduced into the beverage can manufacturing process in the 1960s, and aluminum can sales grew to account for almost all of the beverage can market and almost 90 percent of the soft drink market in 1999 from 2 percent in 1964. This growth can be attributed to ongoing incremental refinements in aluminum can manufacturing and fabrication that facilitated recycling, reduced aluminum consumption per can, and required less energy to produce.
Continued technological refinements in beverage can design have allowed increasingly thinner cans to be manufactured. In 1972, 22 cans could be made from 0.454 kilogram (1 pound) of aluminum; by 1999, 33 cans could be made from the same amount. Design of the aluminum can pop-top is another example in which technological innovation saves materials and energy.
Alloying is the process of adding one or more different elements to a metal for the purpose of enhancing the metal’s properties for a particular application and often results in the use of less material in a particular application. Alloys are often developed to improve performance, to increase efficiency, and (or) to reduce the cost of a product in response to industry or societal needs. Alloys can overcome short-term scarcity of a material by supplying a substitute combination of materials with similar properties and (or) performance characteristics.
Alloyed materials with different properties have been combined to make composites with even more useful properties. Even the orientation or shape of the alloy within the composite has been engineered to change or improve desired physical characteristics. Examples of composites include carbon or glass fibers within plastics. Until 1997, much of the research on composites focused on the needs of the aerospace industry for high-performance lightweight materials. Aluminum/silicon alloys, which deliver such properties either alone or in a matrix, are finding expanded use not only in the aerospace industry, but also in the transportation industry.
The development of coatings technology brings desirable properties to a material while using less of it. In the computer industry, coatings have been important for placing desired electrical properties on the surface of chips and connectors. Many of the rarer elements, such as europium, lanthanum, lithium, strontium, tantalum, and yttrium, have been incorporated into coatings that have been applied to substrates with other desirable properties.
Coatings also are applied to improve surface hardness. Diamond-coated ball bearings have increased wear resistance (figure 11). Silica-based coatings are placed on lightweight plastics to improve abrasion characteristics. Carbon nitride is used to replace diamond as a coating for high-temperature applications. Heat treating stainless steels in nitrogen-rich environments (nitriding) improves the wear resistance (life) of pumps, tools, and valves.
Technological innovations have provided the means for developing manufactured substitutes for minerals and their products, thereby reducing the need for some materials and minerals and conserving resources. The development of synthetic diamond is one prime example of manufactured mineral products.
Diamond has many useful properties. It is the hardest known material, has the highest known thermal conductivity at room temperature, and is transparent over a very wide range of wavelengths. Diamond is also the stiffest and least compressible known material and is inert to most chemical reagents.
Synthetic diamond can be produced by subjecting graphite to very intense pressure (approaching 100 megabars) and high temperatures. This is similar to nature’s method of producing diamond but under controlled conditions that eliminate natural impurities and other imperfections. In 1958, a technology of building diamond structures by adding atoms one at a time to an initial template (a diamond “seed”) was developed. This method proved less costly and energy intensive than pressure-intensive methods. Diamonds produced early in this commercial endeavor had many impurities, but incremental technological improvements over time have resulted in diamond products suitable for many applications.
U.S. apparent consumption of industrial diamond, 90 percent of which is synthetically manufactured, has grown to an estimated 278 million carats in 1999 from 231 million carats in 1995, based on a compound annual growth rate of nearly 5 percent. Constant-dollar prices declined to $4.94 per carat in 1999 from $6.62 per carat in 1995; this indicates the ability of technology to provide industrial diamond at decreasing costs.
Another advance in the use of synthetic diamond technology has been the development of diamond films and coatings that have found application as components of cutting tools, electronic devices, optics, and other applications. Coatings and films add diamond’s unique properties to other materials. The use of synthetic diamond as coatings and films has increased the service life (efficiency) of the resulting composite materials and has also reduced the United States’ reliance on naturally occurring diamond.
Use: “Sewing with the Needle”
Human beings use their expanding technological knowledge to make improved or new materials. Societies traditionally have depended upon these new materials to improve their products and standard of living. New materials have led to advances in agriculture, communications, computer systems, industrial processing, and medical technology (figure 12). Spin-offs from such advanced technologies used for space flight have led to the creation of new materials for consumer products. Technology is often required to design resource-efficient methods to produce these or substitutable materials.
 Figure 12: Changing technology. Advances in materials technology have improved telecommunications and use less material. Old phone photograph courtesy of U.S. Interagency Working Group on Industrial Ecology, Material and Energy Flows, 1999; modern phone photograph courtesy of GadgetCentral, Inc., 2001)
Figure 12: Changing technology. Advances in materials technology have improved telecommunications and use less material. Old phone photograph courtesy of U.S. Interagency Working Group on Industrial Ecology, Material and Energy Flows, 1999; modern phone photograph courtesy of GadgetCentral, Inc., 2001) The copper case study describes how market demand, materials, and technology interrelate (Technological change in the copper industry). The discovery of electricity and its use in communication systems, lighting, and motors generated the demand for materials that could transmit electricity efficiently over long distances. The properties of copper, which include its corrosion resistance, high electrical conductivity, and the introduction of wire-forming technology that allowed copper to be formed into long strands made it the material of choice during the early 20th century for high-capacity transmission lines and household wiring systems.
Countries that have not invested heavily in copper infrastructure for telecommunications may not need to develop such capability because they may be able to go directly to newer technologies. One should not assume that technological development in currently underdeveloped countries places the same demand on resources that was needed to get more-developed countries to where they are today; in other words, past developmental histories should not be extrapolated to estimate future resource demand.
Other materials, however, can be substituted for copper in wiring. Aluminum is used for high-capacity transmission lines. Silicon is used to make fiber-optic cables for telecommunications. New technologies have improved transmission efficiencies for aluminum and fiber-optic cables and have made these commodities competitive.
If the price of a material increases as a result of such factors as increased demand or limited production capacity, then substitution may take place. Examples of substitution include the beverage industry’s use of aluminum for steel cans and plastic for glass bottles. The automotive industry has substituted aluminum for steel body parts, copper for aluminum in radiators, and plastic for chrome bumpers. In each case of substitution, technology has succeeded in developing an alternate form with decreased cost and (or) increased performance benefits.
The search for substitutes can have unintended consequences, however. When the EPA announced in the 1970s its intention to ban the use of asbestos, friction product and high-temperature gasket manufacturers began searching for a substitute that would have the required properties such as a small cross section and high-temperature compressive creep strength and resistance. The industry selected glass fibers as a substitute. Evidence suggests, however, that glass fibers are more costly and less efficient. Glass fibers also have raised environmental concerns that equal or exceed those for asbestos.
The EPA ban on asbestos was partially overturned in 1991 by a ruling of the Fifth Circuit Court of Appeals. Many asbestos products were exempted from the ban; these included but were not limited to asbestos cement pipe, asbestos clothing, disk brake pads, friction materials, gaskets, and vinyl-asbestos tiles.
Recycling
Products that are perceived to have lost their value may be disposed of, recycled to serve as raw materials for manufacturing, remanufactured, or reused. Recycled materials must conform to the same quality and safety standards as manufactured or natural products. As landfills become full and new sites become more difficult to locate, permit, and operate, disposal costs increase. Research is focusing increasingly on developing ways to reuse materials that traditionally have been considered to be waste. The concepts of recycling, redesigning, and remanufacturing, involve turning waste materials into useful products with minimal environmental impact. Although not new, these concepts are becoming more and more important in today’s society. Each of these approaches has the potential of conserving such natural resources as energy and come with significant economic savings and environmental benefits.
Recyclable materials include such items as aluminum cans, automotive parts, construction asphalt and concrete, and copper wire (figure 13). Approximately 120 million metric tons per year (Mt/yr) of materials are recycled in the United States through auto recycling, construction and demolition recycling, and municipal programs, thus saving the equivalent of about 4 months of the Country’s electricity demand. Although recycling can include old scrap (derived from discarded products) and new scrap (purchased from the manufacturing process), only old scrap is included in apparent consumption statistics. Recycling of old and new scrap was estimated to account for approximately 63 percent of the apparent supply of lead, 55 percent of steel, 50 percent of titanium; and between 25 and 40 percent aluminum, copper, Magnesium, nickel, tin, and zinc in 1998. The aluminum and the copper case studies show how technology has influenced the growth of recycling and increased the availability of resources from more-diverse source (Technological change in the aluminum industry, Change in the copper industry]).
Recycling conserves embodied energy and reduces waste. This is also the goal of mandated recycling. Technology, however, can often address obstacles to recycling. For example, direct-reduced iron (DRI) technology was developed by the U.S. steel industry in the 1970s to alleviate the problems that resulted from the use of 100-percent scrap iron in electric furnace steel production. When scrap is fed to electric furnaces to make steel, it contains residual, often inseparable elements other than iron. As recycling continues, the weight percentage of these alloying elements tends to accumulate above steel specification limits. When mixed with scrap, the relatively pure DRI dilutes the undesirable content of the resulting mixture, which keeps the steel within specifications. The elements that accumulate in electric furnace steelmaking are, in fact, conserved through the use of DRI because they are removed and placed back into useful applications more quickly. DRI sustains the demand for alloy scrap.
The DRI process uses natural gas to reduce iron oxides in pellets to elemental iron. In 1999, the feed to U.S. electric furnaces was about 70 percent scrap and 30 percent DRI. DRI is a complement for scrap, and the dynamics of scrap prices affects the profitability of DRI operations. Most of the DRI production facilities are in developing countries because of the high cost of its alternative, steel scrap.
Recycling technology has responded to environmental regulations by reducing the discharge of toxic materials, such as arsenic, cadmium, lead, and mercury, into the environment and reduced their use in manufacturing processes and products. For example, legislation has mandated the recovery of lead from lead-acid storage automotive batteries since 1996. Technology was developed so that about 76 percent of refined lead produced in the United States in 1998 was recovered from recycled scrap, a major source of which was spent lead-acid storage batteries.
Recycling of cadmium from spent nickel-cadmium storage batteries also has grown during the 1990s. Research during this time has allowed scientists to document the toxicity of arsenic (Health effects of arsenic) and mercury (Health effects of mercury) in the environment and on human health, and regulations have been implemented to limit their effects. Technology has been developed to recover and extract mercury more efficiently from its products which include control instruments, dental amalgams, electrolytic refining laboratory wastes, fluorescent and vapor lamps, measuring devices, spent batteries, and switches.
One of the characteristics of aluminum is that it is easily recyclable. The aluminum recycling (secondary) industry has processed scrap from the primary aluminum industry since about 1904. The amount of energy required to produce 1 t of recycled aluminum is about 5 percent of the energy required to produce 1 t of primary aluminum from bauxite. Before World War II, little primary aluminum was produced. Recovery of aluminum scrap was insignificant because the supply of scrap was limited. As the aluminum industry expanded during the War and entered new markets, recycled aluminum production also increased because of favorable economics, increased supply of aluminum scrap, and proven product performance. Some technical advances in alloying and die casting during the Second World War were developed specifically for the secondary aluminum industry. Technology developed during the 1950s allowed improved material separation of aluminum scrap from junked automobiles. Recycling of aluminum products increased dramatically in the 1970s when the aluminum beverage can began to be widely used. In 1999, the United States produced about 3.8 Mt of primary aluminum metal and recovered about 3.5 Mt from purchased scrap.
Recovery of obsolete materials from the burgeoning electronics industry continues to grow (figure 14). Table 2 lists materials recovered from U.S. electronics recyclers. The amount of recycling of obsolete electronic products to recover reusable components, such as glass, metals, and plastics, is increasing. The reuse of components and refurbishment of computers lengthens their life spans. As electronic products advance technologically, however, the amount of precious metals used in their components decreases, and as the rate of advance accelerates, old computer parts are worth less. Scrap that has lower value coupled with increasing labor, plant, and regulatory costs could result in decreased recycling. Nevertheless, recycling may continue to increase as manufacturers use, for example, management and organizational technologies to develop better ways to identify types of plastics, to design for greater ease in dismantling, and to develop leasing programs that include the return of electronic products to the manufacturer or retail distributor. Recycling also can increase through more effective collection methods and legislation that mandates refundable deposits at the time of purchase and through take-back programs or bans on landfill disposal.
| Type of material | 1997 | 1998 |
| Glass | 11,600 | 13,200 |
| Plastic | 3,700 | 6,500 |
| Metals: | ||
| Aluminum | 3,900 | 4,500 |
| Steel | 14,500 | 19,900 |
| Copper | 4,300 | 4,600 |
| Combined precious metals (gold, palladium, platinum, silver) | 1 | 1 |
| Other | 3,100 | 3,600 |
| Total | 41,100 | 52,300 |
| Adapted from National Safety Council, 1999, p. 36; Sean Magaan, Noranda Inc., Micro Metallics Corp., oral commun., 1999 | ||
Technologies are being developed to recover materials from such nontraditional sources as mine tailings. Magnesium, which is the lightest of all structural metals and a critical constituent in some aluminum-base alloys and castings, is an important metal in the aerospace and automotive industries. Most of the world’s magnesium originates from brines (including seawater), dolomites, and salt deposits. A new low-cost extractive technology to recover magnesium from serpentinite tailings, which is a waste material generated from the mining for asbestos, is being evaluated in Australia, Canada, Russia, and the United States. Extraction of magnesium by recycling tailings through the process offers the advantage of recovering a metal from materials that, until recently, were considered to be waste and economic and environmental liabilities. Recovery of such material reduces production from other ore sources and effectively extends the life of natural resources from which magnesium is recovered.
In Canada, the first commercial venture to recover magnesium from wastes generated from more than 100 years of asbestos mining began production in late 2000. When fully operative, the Magnola facility will be the world’s leading source of magnesium and the first to use an innovative but still commercially unproven technology. Development of the extractive process took more than 10 years and millions of dollars to develop. The resource is derived from more than 250 Mt of former waste material—a legacy of more than 100 years of asbestos mining. Resources at the $500 million facility will be sufficient to produce 63,000 t/yr of magnesium metal for about 300 years.
Innovations in recycling have reduced the amount of industrial and municipal waste, thereby supplementing the supply of “new” materials and reducing the amount of material that occupies landfills. For example, Chaparral Steel Company of Midlothian, Texas, is taking automobile recycling one step farther by using an innovative flotation technology to separate the various materials in automobile shredder residue (ASR). ASR is material that is leftover following the processing of scrap automobiles and typically includes aluminum, glass, magnesium, other nonferrous metals, polyvinyl chloride (PVC) and other plastics, and rubber, all of which are potentially recyclable. This technology is based on that used in the mining industry to recover the valuable components of mineral ores. Historically, the material was dumped in landfills because of the high costs associated with separating the mixture of material into its individual components. In the United states an estimated 3 to 5 Mt/yr of ASR is generated (Argonne National Labs). Nonchlorinated plastics may be used as a highly efficient and clean fuel source rather than being placed in landfills. Chaparral Steel anticipates that the glass can be remelted or used as roadbed material or as an abrasive for sanding devices. Potentially, this new technology could be used for mining municipal landfills.
Remanufacturing
Remanufacturing is the process of disassembling a product and then cleaning, repairing, replacing, and reassembling it such that only a small part of the original product is not returned to service. Reuse of materials reduces reliance on primary mineral resources. The origins of remanufacturing can be traced to the 1920s and 1930s with the emergence of mass production and standardization of such products as the automobile and the refrigerator. Economic and resource constraints brought about by the Depression and World War II resulted in a major period of growth for remanufacturing during a time when a scarcity of such raw materials as steel drove the need to reuse durable goods. Although estimates vary on the size and scope of the remanufacturing industry, an analysis by Boston University in 2000 found 73,000 remanufacturing firms operating in the United States. These firms represent $53 billion in annual sales and employ 480,000 people.
Extending product life through remanufacturing is an increasingly important component of conserving the Earth’s natural resources. Remanufacturing offers greater advantages than recycling. For example, if an automobile’s engine is recycled, then the steel is saved from the landfill space and could be used to produce another item requiring steel. Remanufacturing, however, offers another alternative. This process retains most of the value added to the product, which includes the cost of energy, labor, and raw materials, when it was first manufactured. Rebuilt engines, for instance, require only 50 percent of the energy and 67 percent of the labor needed to produce a new engine.
A scientist at the Energy Systems Division of Argonne National Laboratory estimated that remanufactured products conserve the equivalent of 68 million barrels per year of oil worldwide, which is equivalent to the energy content of gasoline to operate about 6 million passenger vehicles for 1 year. In addition to saving energy and other raw materials, remanufacturing reduces the potential for the release of toxic materials, which include gases, some of which contribute to the load of greenhouse gases in the atmosphere (Atmosphere layers) and is thought to extend the life of landfills.
Remanufacturing also results in significant economic benefits. Purchasing a remanufactured product can cost consumers from 50 to 75 percent less than a new product. Technological advancements in product composition and design have lengthened the life of products and improved the ability to refurbish them. As part of their business plans, many companies also are designing products that can be more easily remanufactured.
Signature-analysis technology may be used throughout the automotive industry by 2003. This type of analysis is a procedure that predicts the remaining life of major product subcomponents to maximize their use. Improved cleaning methods and equipment that reduce disposal rates and environmental impact by using less solvent may be developed by 2005. Additional technology goals could be established that set targets for improved auto-salvage techniques, processing methods, testing, and after-market engineering with the goal of approaching zero waste by 2020.
Other industries also have a history of remanufacturing. Xerox Corporation, of Stamford, Connecticut, for example, has been reclaiming metals from its product components since 1967 and “unofficially” has been accepting trade-in machines from customers for almost as many years. In 1990, the company initiated its Environmental Leadership Program with the goal of producing waste-free products. Remanufactured machines are a significant and profitable part of the company’s product line. New products are designed to be remanufactured, reused, and (or) recycled. In 1997, Xerox remanufactured equipment from more than 30,000 t of returned machines, and customers returned 65 percent of all empty copy and print cartridges to the company for recycling.
Reuse
One of the most dramatic reductions in waste and conservation of resources can be made by product reuse, in which the form of the product is retained and the product is reused for the same purpose as during its life cycle. Examples include refillable drink bottles, refurbished computers, and used automobile fenders and bumpers. Reuse also includes finding new uses for “used-up” products, such as automotive tires, which can be used as mooring cushions on harbor docks. Reuse has the added benefits of conserving landfill space, reducing waste generation, and saving the energy and additional material that would be needed to form these materials into new shapes. Every time a product is reused, most of the energy used and the emissions produced in its original manufacturing and processing are, in a sense, retained. Moreover, technological advances in product design and collection and increases in productivity through automation have improved the efficiencies in product reuse.
Conclusions Predictions of resource scarcity occur periodically. This study shows how the implementation of technology, which is the organization of energy, knowledge, labor, and materials, has historically addressed scarcity concerns. Although we know intuitively that resources are finite, the historical evidence suggests that resource availability has grown consistently and that real prices (by some measures) for materials during the past 100 years have trended downward or remained relatively steady. This has taken place in the face of declining ore grades, increasing demands by a population that is growing and has increasing lifestyle expectations, and mining resources that are increasingly difficult to access because of their depth, geologic complexity, remoteness, and (or) susceptibility to adverse political and social actions. Productivity improvements and an expanding diversity of resources from which materials can be profitably extracted and manufactured have come about through advances in technology. Technological developments are driven by the desire to supply material at the highest possible profit. (Technology and the materials cycle)
Further Reading
- Airborn Electronics Company, 2001. Examples of Airborn Electronics work. Accessed March 22, 2001.
- Altschuller, Allison, 1997. Automobile recycling alternatives—Why not? Accessed June 5, 2000.
- Aluminum Association, Inc., 1985. Aluminum recycling casebook: Washington, D.C., Aluminum Association, Inc., December 1985, p. 7-9.
- Aluminum Association, Inc., 1999. Aluminum facts at a glance. Accessed June 1, 2000.
- Amey, E.A., 2000. Gold recycling—A materials flow study. Accessed December 24, 2000.
- ASARCO Incorporated, 1999. Asarco—A reliable source for refined copper. Accessed July 23, 2003.
- Asbestos Institute, 1995. (part2).html In search of responsible rule-making. Accessed July 25, 2000.
- Ascough, Graham, 1999. Geophysical characteristics of volcanogenic massive sulfide deposits, in Lowe, Carmel, Thomas, M.D., and Morris, W.A., eds., Geophysics in mineral exploration—Fundamentals and case histories: Geological Society of Canada Short Course Notes, v. 14, p. 41-62.
- Automotive Parts Rebuilders Association, 2000a. Remanufacturing vision statement. Accessed August 24, 2000.
- Automotive Parts Rebuilders Association, 2000b. Remanufacturing—An answer to global warming. Accessed August 25, 2000.
- Bliss, J.D., ed., 1992. Developments in mineral deposit modeling: U.S. Geological Survey Bulletin 2004, 168 p.
- Buckingham, D.A., and Ober, J.A., 2002. Sulfur statistics, in Kelly, Thomas, Buckingham, David, DiFrancesco, Carl, Porter, Kenneth, Goonan, Thomas, Sznopek, John, Berry, Cyrus, and Crane, Melissa, eds., Historical studies for mineral commodities in the United States. Accessed June 4, 2001.
- Buckingham, D.A., and Plunkert, P.A., 2002. Aluminum statistics, in Kelly, Thomas, Buckingham, David, DiFrancesco, Carl, Porter, Kenneth, Goonan, Thomas, Sznopek, John, Berry, Cyrus, and Crane, Melissa, eds., Historical studies for mineral commodities in the United States. Accessed June 4, 2001.
- Buckingham, D.A., and Searls, J.P., 2002. Potash statistics, in Kelly, Thomas, Buckingham, David, DiFrancesco, Carl, Porter, Kenneth, Goonan, Thomas, Sznopek, John, Berry, Cyrus, and Crane, Melissa, eds., 2001, Historical studies for mineral commodities in the United States. Accessed June 4, 2001.
- Centre de Recyclers Universel, 2001. The automotive recycling and dismantling process. Accessed March 23, 2001.
- USGS, 2005. Technological Advancement -- A Factor in Increasing Resource Use. U.S. Geological Society (By David Wilburn, Thomas Goonan, and Donald Bleiwas; with an Introduction by Eric Rodenburg).
|
This article contains information that was originally published by the USGS. Topic editors and authors for the Encyclopedia of Earth have edited its content and added new information. The use of information from the USGS should not be construed as support for or endorsement by that organization for any new information added by EoE personnel, or for any editing of the original content. |