Heat

An object’s kinetic energy can be classified as internal or external. For example, a falling coin has a certain external kinetic energy that is related to its overall mass and to its velocity as it falls. The coin is also composed of particles that, like all particles, are moving in a random way, independent of the overall motion (or position) of the coin. The particles in the coin are constantly moving, colliding, changing direction, and changing their velocities. The energy associated with this internal motion is internal kinetic energy (Figure 1).
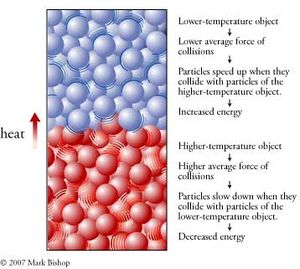
Heat is a general term that represents the amount of internal kinetic energy an object contains. The internal kinetic energy of an object can be increased by putting it in contact with another object at a higher temperature. Temperature is proportional the average internal kinetic energy of an object, so higher temperature means a greater average internal energy for the particles within the object. The particles in a higher-temperature object collide with other particles with greater average force than the particles of a lower-temperature object. Thus collisions between the particles of two objects at different temperatures cause the particles of the lower-temperature object to speed up, increasing the object’s energy, and cause the particles of the higher-temperature object to slow down, decreasing this object’s energy. In this way, energy is transferred from the higher-temperature object to the lower-temperature object. We call energy that is transferred in this way heat. The energy that is transferred through an object, as from the bottom of a cooking pan to its handle, is also called heat. Heat is the energy that is transferred from a region of higher temperature to a region of lower temperature as a consequence of the collisions of particles (Figure 2).
Further Reading
- This article is an excerpt from the preparatory chemistry text An Introduction to Chemistry by Mark Bishop.