Pressure
p = F/A
where: p is the pressure, F is the force and A is the area.
The System Internationale (SI) unit for pressure is the pascal (Pa), equal to one newton per square meter (N·m–2 or kg·m–1·s–2). It was given that SI name in 1971.
Contents
Earth's Atmospheric pressure
The atmospheric pressure at any given point in Earth's atmosphere is the downward force per unit are exerted upon a horizontal surface at that point by the weight of air above that surface.
Atmospheric pressure at sea level will vary with geographic location, the temperature and humidity of the air and with the weather conditions. In fact, a change in the sea level atmospheric pressure usually indicates an upcoming change in the weather. Since air temperature and humidity as well as the weather change with the annual seasons (i.e., winter, spring, summer and fall), the sea level atmospheric pressure also changes with the seasons.
Standard value of atmospheric pressure at sea level
In 1954, the 10th Conférence des Poids et Mesures (CGPM) adopted a standard atmosphere for general use and defined it as being precisely 1,013,250 dynes per square centimeter (101,325 Pa). This value was intended to represent the average atmospheric pressure at the average sea level at the latitude of Paris, France, and as a practical matter, truly reflects the average sea level pressure for many of the industrialized nations (those with latitudes similar to Paris).
The International standard atmosphere (ISA) as used by International Civil Aviation Organization (ICAO) is also defined as being 101,325 Pa at sea level. The U.S. Standard Atmosphere also defines the sea level atmospheric pressure as being 101,325 Pa.
In chemistry, the original definition of "Standard Temperature and Pressure" by the International Union of Pure and applied Chemistry IUPAC) was a reference temperature of 0 °C (273.15 K) and pressure of 101,325 Pa. However, in 1982, the IUPAC recommended that for the purposes of specifying the physical properties of substances, the "standard pressure" should be defined as 100,000 Pa (1 bar).
However, the sea level atmospheric pressure of 101,325 Pa (as defined by the CGPM, the ICAO and also the IUPAC prior to 1982) continues to be very commonly used.
Various units of pressure
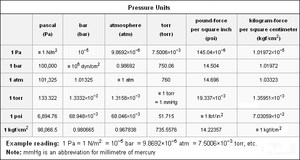
Most of the various units of pressure and their equivalents to each other are listed in the table below:There is no consensus in the technical literature about whether the name/unit torr should begin with a capitol letter - as in "Torr" or simply "torr". Both the United Kingdom's National Physical Laboratory (see Pressure Units) and New Zealand's Measurement Standards Laboratory (see Barometric Pressure Units) use "torr" as the name and as the symbol. An extensive search of the website of the U.S. National Institute of Standards and Technology (NIST) found no such clear-cut definitions. Therefore, this table uses "torr" as both the name and the symbol.Absolute pressure versus gauge pressure
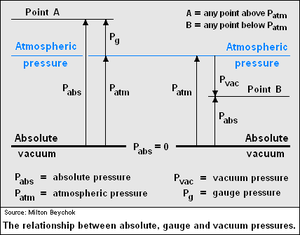
Bourdon tube pressure gauges, vehicle tire gauges and many other types of pressure gauges are zero referenced to atmospheric pressure, which means that they measure the pressure above atmospheric pressure. However, absolute pressures are zero referenced to a complete vacuum. Thus, the absolute pressure of any system is the gauge pressure of the system plus the local atmospheric or ambient pressure. The adjacent diagram illustrates the relationship between gauge and absolute pressure for any pressures above atmospheric pressure.An example of the difference between gauge and absolute pressure is the air pressure in a vehicle tire. A tire pressure gauge might read 220 kPa as the gauge pressure, but that means the pressure is 220 kPa above atmospheric pressure. Since atmospheric pressure at sea level is about 101 kPa, the absolute pressure in the tire is therefore about 321 kPa.
In technical writing, this would be written as a gauge pressure of 220 kPa or as an absolute pressure of 321 kPa. Where space is limited, such as on pressure gauge dials, table headings or graph labels, the use of a modifier, such as kPa (gauge) and kPa (absolute) or kPa-gauge and kPa-absolute, is strongly encouraged. Gauge pressure is also sometimes spelled gage pressure. This discussion of modifiers also applies to the other pressure units such as bar, torr, atmosphere, etc.
Negative or vacuum pressures
While pressures are generally positive, when describing a system operating at a pressure below atmospheric pressure (a partial vacuum), the pressure (as shown in the asjacent diagram) may be expressed in terms of how much it is below atmospheric pressure or in terms of how much it is above a complete vacuum. For example, if the atmospheric pressure is 101 kPa, point B in the diagram might be expressed as being 45 kPa of vacuum, meaning it is 45 kPa below atmospheric pressure. It might also be expressed as 56 kPa (absolute) meaning that it is 56 kPa above zero pressure. In technical writing, it is very important to make clear how any pressures below atmospheric pressure are expressed.
A statement such as the system operates at a vacuum of 100 torr will lead to confusion because it might mean an absolute pressure of 100 torr or a pressure of −100 torr (i.e., 100 torr below an atmospheric pressure of 760 torr).
References
- IUPAC definition of "pressure".
- IUPAC definition of "Standard Pressure".
- Search Results 1 and 2 ,From the website of the National Physics Laboratory, United Kingdom.
- Arnold Ivan Jones and Cornelius Wandmacher (2007), Metric Units in Engineering:Going SI, Revised Edition, American Society of Civil Engineers, page 147, ISBN 0-7844-0070-9.
- BIPM (CGPM) Definition of the "Standard Atmosphere"
- International Civil Aviation Organization, Manual of the ICAO Standard Atmosphere, Doc 7488-CD, Third Edition, 1993, ISBN 92-9194-004-6.
- U.S. Standard Atmosphere, 1976, Scroll to pdf page 28 of 241 pdf pages.