Future change in processes and impacts on Arctic biota
This is Section 9.3.4 of the Arctic Climate Impact Assessment
Lead Author: Harald Loeng; Contributing Authors: Keith Brander, Eddy Carmack, Stanislav Denisenko, Ken Drinkwater, Bogi Hansen, Kit Kovacs, Pat Livingston, Fiona McLaughlin, Egil Sakshaug; Consulting Authors: Richard Bellerby, Howard Browman,Tore Furevik, Jacqueline M. Grebmeier, Eystein Jansen, Steingrimur Jónsson, Lis Lindal Jørgensen, Svend-Aage Malmberg, Svein Østerhus, Geir Ottersen, Koji Shimada
Table 9.4 summarizes the potential physical oceanographic changes in the Arctic based on the projected changes in the atmospheric forcing functions (Table 9.1) and potential future sea-ice conditions discussed in Chapter 6 (Future change in processes and impacts on Arctic biota). Table 9.10 summarizes the potential long-term ecological changes in the marine system that are considered likely to arise as a result of these physical changes. The time frames for these changes to the biological system are addressed in this section by trophic level and by region where appropriate. The most pronounced physical changes are likely to include a substantial loss of sea ice, an increase in air and sea surface temperature, and changes in the patterns of wind and moisture transport.
|
Table 9.10. Potential long-term ecological trends due to climate warming. Unless otherwise specified these projected changes are very likely to happen. | |||||
|
Phytoplankton |
Zooplankton |
Benthos |
Fish |
Marine mammals and seabirds | |
|
Distribution |
Increased spatial extent of areas of high primary production in the central Arctic Ocean. |
Southern limit of distribution for colder water species to move northward. Distribution of more southerly species to move northward. |
Southern limit of distribution for colder water species to move northward. Distribution of more southerly species to move northward. |
Southern limit of distribution for colder water species to move northward. Distribution of more southerly species to move northward. Timing and location of spawning and feeding migrations to alter. |
Poleward shift in species distributions. |
|
Production |
Increased production in central Arctic Ocean, and Barents and Bering Sea shelves. |
Difficult to predict, will depend on the timing of phytoplankton production and seawater temperatures. |
Difficult to predict, will partly depend on the degree of match/ mismatch between phytoplankton/ zooplankton production and on water temperature. Production by shrimp and crab species may decline. |
Wind-driven advection patterns of larvae may be critical as well as a match/mismatch in the timing of zooplankton production and fish larval production. |
Dramatic declines in production by ice-associated marine mammals and increases by more temperate species. Seabird production likely to be mediated through forage availability, which is unpredictable. |
|
Species composition/ diversity |
Dependent on mixing depth: shallow mixing favors diatoms, intermediate depth mixing favors Phaeocystis, deep mixing may favor nanoflagellates. |
Adaptable arctic copepods, such as Calanus glacialis, may be favored. |
Cold-water species may decline in abundance along with some clams and crustaceans, while warm water polychaetes, blue mussel (Mytilus edulis), and other types of benthos may increase. |
Cod, herring, walleye pollock, and some flatfish are likely to move northward and become more abundant, while capelin, polar cod, and Greenland halibut will have a restricted range and decline in abundance. |
Declines in polar bear, and in ringed, harp, hooded, spotted, ribbon, and possibly bearded seals. Increased distribution of harbour seals and grey seals. Possible declines in bowhead, narwhal, grey, and beluga whales. Ivory gulls and several small auk species are likely to decline while other changes in bird populations are unpredictable. |
Changes in the distribution of many species, ranging from phytoplankton to whales, are very likely to occur. The main habitat changes affecting marine mammals and seabirds include a reduction in sea ice, changes in snow cover, and a rise in sea level. Phenological changes, species replacements, and changes at lower trophic levels are also likely to have a strong influence on upper trophic level species.
Contents
Primary production (9.3.4.1)
Changes in sea-ice, water temperature, freshwater input, and wind stress will affect the rate of nutrient supply through their effect on vertical mixing and upwelling. Changes in vertical mixing and upwelling will affect the timing, location, and species composition of phytoplankton blooms, which will in turn affect the zooplankton community and the productivity of fish.
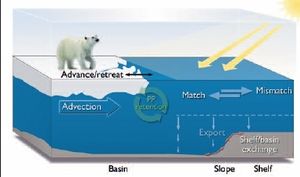
Changes in the timing of the primary production will determine whether this production is utilized by the pelagic community or is exported and utilized by the benthos (Box 9.10). The retention to export ratio also depends upon the advection and temperature preferences of grazing zooplankton, which together determine the degree of match or mismatch between primary and secondary production. The projected disappearance of seasonal sea ice from the Barents and Bering Seas (and thus elimination of ice-edge blooms) implies that these areas would have blooms resembling those of more southerly seas. The timing of these open ocean blooms in the Barents and Bering Seas will then be determined by the onset of seasonal stratification, again with consequences for a match/mismatch in timing with zooplankton.
|
Box 9.10. Effects of a variable ice edge on key biological processes affecting carbon flux on an arctic shelf Primary production (PP) occurs in the euphotic zone when light and nutrient conditions allow. This primary production may be retained by recycling within the euphotic zone or exported to deeper waters and be available for the benthos.The efficiency of retention is strongly determined by the occurrence of a match (where zooplankton are available to graze and recycle the primary production) or mismatch (where zooplankton are not present in sufficient numbers and primary production sinks out of the euphotic zone to be grazed by the benthos). Zooplankton densities may be affected by advection in certain shelf locations such as the Barents and Chukchi Seas. Additional concerns involve sequestration of carbon in shelf, slope, and basin sediments, and exchange processes that act to move carbon from one regime to another (red arrows).The location of the ice edge, where much primary production occurs, relative to topography (e.g., the shelf break and slope) strongly impact upon all of these processes. Under climate change scenarios, the ice edge will retreat further and faster into the basin, thus increasing the export of PP first to the slope and then to the abyssal ocean (E.C. Carmack, personal communication, 2004). |
Removal of light limitation in areas presently covered by multi-year sea ice is likely to result in a two- to five-fold increase in primary production, provided wind mixing is sufficient to ensure adequate nutrient supply. Moreover, earlier melting in the seasonal sea-ice zone is likely to enhance annual primary production by extending the growing season. The actual outcome in terms of annual production, however, is highly dependent upon regional and local changes in upwelling, wind-driven vertical mixing, and freshwater supply from sea ice and rivers. Note, for example, that it takes only a small increase in salt stratification (i.e., a decrease in surface salinity) to offset the effect of increased winds on vertical mixing. Regional cooling, as projected by some of the ACIA-designated models, would result in the opposite effects to those of the warming scenarios described in the rest of this section.
The disappearance of sea ice from the Barents Sea is likely to result in a more than doubling of the present levels of primary production, especially in the northernmost part. This is a consequence of a deeper wind-mixed layer and an increased vertical supply of nutrients from the underlying Atlantic water. Predicting changes in the timing of the spring bloom requires a better understanding of, and capability of modeling, the combined effects of ice-edge retreat and stability in the position of the Polar Front. To the south of the Polar Front, the absence of sea ice will reduce stratification thereby delaying the spring bloom until the onset of thermal stratification and the development of the seasonal surface mixed layer. North of the Polar Front, however, the timing of the spring bloom is strictly tied to light availability. At present, the spring bloom in the northern Barents Sea must await the retreat of the marginal ice zone for adequate light levels. In the absence of sea ice, the spring bloom is likely to occur earlier, and is very likely to occur earlier than in the region to the south of the Polar Front.
Primary production on the Bering Shelf is also likely to be enhanced if it becomes permanently ice-free, primarily due to an extended growing season and continuous upwelling of nutrient-rich water along the highly productive zone associated with the Bering Shelf break. More intense wind and more arid conditions at and near the Gobi and Takla Makan deserts in northeast Asia will possibly lessen the impact of iron control in the Northeast Pacific and the eastern Bering Sea.
In the shelf seas of the Arctic Ocean (e.g., the Kara, Laptev, East Siberian, and Beaufort Seas), a significant increase in nutrient supply is very likely to happen when the edge of the permanent ice pack retreats beyond the shelf break. This is very likely to trigger the onset of shelf-break upwelling and the delivery of nutrient-rich offshore waters to shallow shelf regions, perhaps more than doubling present levels of productivity.
In the central Arctic Ocean, two additional conditions of sea-ice retreat are important to primary production: the disappearance of sea-ice cover in summer and the regional appearance of open water areas in winter (e.g., north of Svalbard and northeast of the Chukchi Sea). In open water areas during summer, productivity is likely to increase due to increased wind mixing and nutrient re-supply. Within areas regionally open in winter, additional nutrients are likely to be supplied through the combined effects of wind stress and convective mixed layer deepening. It is possible that these two types of area will be as productive as is currently the case in their southern counterparts (the Greenland and deep Bering Seas, respectively). Before the development of these two distinctive conditions, areal primary production is likely to increase as the number and size of leads in the multi-year ice increase.
Surface mixed-layer depth is likely to have a strong impact on phytoplankton community structure, particularly in the Nordic Seas. Regions where the seabed or the depth of mixing (due to ice melt or river inflow) is less than about 40 meters (m) are likely to favor diatom blooms. Deeper mixing, to about 80 m, is likely to favor Phaeocystis. Thus, unless there is an increase in freshwater input, stronger winds are likely to result in Phaeocystis becoming more common than at present. This is possible in Atlantic water to the south of the Polar Front. If the surface mixed layer in the Atlantic water extends beyond about 80 m, it is possible that a low-productive community dominated by nanoflagellates would be favored, as currently occurs in the off-shelf parts of the Southern Ocean[1]. This implies little transfer of carbon to herbivores (Herbivory) and sediments because the grazers would be largely ciliates[2].
Zooplankton production (9.3.4.2)
Any northward extension of warm water inflows is likely to carry with it temperate zooplankton, for instance into the Siberian Shelf Seas and the Bering Shelf[3]. Such inflows are likely to include gelatinous plankton in summer and autumn[4]. Ice fauna such as the large amphipods will suffer massive loss of habitat if multi-year ice disappears. The possibility of increased transport of cold water on the western side of the North Atlantic could bring cold-loving zooplankton species farther south. Correspondingly, the southern limit of distribution of northern species may shift northward on the eastern side of the North Atlantic and southward on the western side, as indicated by zooplankton studies over the last 40 years[5].
If the Siberian Shelf Seas become warmer in the future, it is possible that Calanus finmarchicus will thrive and multiply throughout the area as a whole, rather than being restricted to the Siberian Shelf water as currently occurs. There is, however, risk of a mismatch with phytoplankton blooms in that earlier melting will cause earlier stratification and, thus, an earlier bloom. However, if sea ice is absent during summer and autumn, there will be deeper vertical mixing, making the system more like that of the southern Barents Sea, with later blooms, albeit dependent on stratification caused by freshwater inputs from rivers. If water [[temperature]s] in the Siberian Shelf Seas stay lower than presently occur in the southern Barents Sea, the development of C. finmarchicus is likely to be retarded.
Grazing versus sedimentation
If a mismatch occurs in the timing of phytoplankton and zooplankton production due to early phytoplankton blooms, the food web will be highly inefficient in terms of food supply to fish and export production[6]. Export production and protozoan biomass are likely to increase.
A match with phytoplankton blooms can be achieved by arctic copepods, such as C. glacialis, which can adjust its egg production to the development of the phytoplankton bloom whether early or late in the season. This may also pertain to other important copepods in arctic waters. If so, actively grazing zooplankton "for all seasons" are very likely to exist for any realistic climate change and thus future ratios of grazed to exported phytoplankton biomass in the Arctic Ocean are unlikely to be much different to those at present.
Fish versus zooplankton
The crucial issue concerning the effects of climate change on zooplankton production is likely to be related to the match versus mismatch between herbivorous zooplankton and fish. The extent to which commercially valuable fish will migrate northward and the extent to which they will be able to utilize early developing populations of C. glacialis along the Siberian Shelf are unknown. A worst-case scenario would be a mismatch resulting in starving and, ultimately, dying fish in a summer ecosystem characterized by protozoans and unsuccessful, inflexible copepods such as C. finmarchicus.
Benthos (9.3.4.3)
Future fluctuations in zoobenthic communities are very likely to be related to the temperature tolerance of the animals and to future water temperatures. While the majority of boreal forms have planktonic larvae that require a fairly long period to develop to maturity, arctic species do not[7]. Thus, boreal species should be quick to spread in warm currents in periods of warming, while the more stenothermal arctic species (i.e., those able to live within a narrow temperature range only) will perish quickly. In periods of cooling, the arctic species, with their absence of pelagic stages are very likely to slowly follow the warmer waters as they recede. Boreal species that can survive in near-freezing water could remain within the cooler areas.
From the prevailing direction of warm currents in the Barents Sea, shifts in the geographical distribution of the fauna should be quicker and more noticeable during periods of warming than periods of cooling. Any change in the abundance or biomass of benthic communities is most likely to result from the impact of temperature on the life cycle and growth rate of the various species. If warming occurs within the Barents Sea over the next hundred years, thermophilic species (i.e., those capable of living within a wide temperature range) will become more frequent. This is likely to force changes in the zoobenthic community structure and, to a lesser extent, in its functional characteristics, especially in coastal areas.
The highly productive region to the north of the Bering Strait is likely to undergo changing hydrographic conditions, which in turn are likely to result in changes to the dominant species[8]. The hydrography of the St. Lawrence Island polynya region and the Anadyr region is ultimately related to the northward transport of water through the Bering Strait. Because the latter is related to variations in the AO, the future of the northern Bering Shelf is very likely to be closely related to variations in these oscillations[9]. If AO+ conditions predominate in the future, it is likely that the flow of Bering Water into the Arctic Ocean will be small, resulting in a reduction in northward transport of water south of St Lawrence Island.
Because the Gulf of Anadyr "cold pool" is maintained by sea-ice production/brine formation in the St Lawrence Island polynya, an enhanced and more energetic polynya resulting from warming is likely to maintain a chemostat-type bloom system[10], allowing a longer growing season and higher levels of production.
Fish production (9.3.4.4)
Understanding how climate variability affects individual fish populations and fisheries and how the effects differ between species is extremely important when projecting the potential impacts of climate change. Projections of the response of local marine organisms to climate change scenarios have a high level of uncertainty. However, by using observations of changes in fish populations due to past climate variability it is possible to predict some general responses.
Climate change can affect fish production through a variety of means. Direct effects of temperature on the metabolism, growth, and distribution of fish could occur. Food web effects could also occur, through changes in lower trophic level production or in the abundance of top-level predators, but such effects are difficult to predict. However, it is expected that generalist predators are more adaptable to change than specialists. Fish recruitment patterns are strongly influenced by oceanographic processes such as local wind patterns and mixing and by prey availability during early life stages, which are also difficult to predict. Recruitment success could be affected by changes in the time of spawning, fecundity rates, survival rate of larvae, and food availability.
General trends in distribution and production
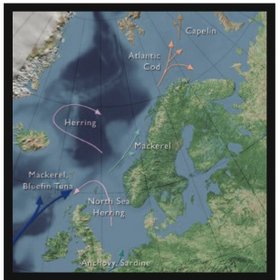
Poleward extensions of the distribution range for many fish species are very likely under the projected climate change scenarios (see Fig. 9.30 and Box 9.11). Some of the more abundant fish species that would be very likely to move northward under the projected warming include Atlantic and Pacific herring and cod, walleye pollock in the Bering Sea, and some of the flatfishes that might presently be limited by bottom temperatures in the northern areas of the marginal arctic seas. The southern limit of colder-water fishes such as polar cod and capelin would be very likely to move northward. The Greenland halibut is also likely either to shift its southern boundary northward or restrict its distribution more to continental slope regions. Salmon, which show high fidelity of return to natal streams, might possibly be affected in unknown ways that relate more to conditions in natal streams, early marine life, or feeding areas that might be outside the Arctic.
|
Box 9.11. Climate impact on the distribution of fish in the Norwegian and Barents Seas An increase in water temperature of 1 to 2 ºC in the Atlantic part of the Norwegian and Barents Sea is very likely to result in a change in distribution for several species of fish. However, in both seas there are fronts between the warm Atlantic water and the cold arctic water masses, whose position is partly determined by bottom topography. How these fronts may move in future is addressed in Section 9.2.5.4 (Future change in processes and impacts on Arctic biota). Previous experience of how fish react to changes in water temperature in the Barents Sea may be used to speculate about future changes.The most likely impact of an increase in water temperature on some commercial fish species in shown in Fig. 9.30. Capelin is very likely to extend its feeding area north and northeastward. During summer it might feed in the Arctic Basin and migrate to the Kara Sea. Whether the capelin maintain their spawning ground along the coast of northern Norway and the Kola Peninsula is unknown.They may possibly move eastward, and may even spawn along the west coast of Novaya Zemlya. Cod is also likely to expand its feeding area eastward, especially as capelin is its main food source. As cod is demersal (i.e., a near-bottom fish), it is not likely to migrate north of the Barents Sea and into the deep Arctic Basin. Haddock will probably follow the same track as cod, but as at present is likely to remain further south than cod. In the Norwegian Sea, herring is likely to return to the feeding and overwintering area used before 1964 (see Box 9.8), but is likely to maintain the same spawning areas along the Norwegian coast. Mackerel (Scomber scombrus) and blue whiting (Micromesistius poutassou) are likely to migrate northeast to the Barents Sea.The mackerel and blue whiting will then compete with the other pelagic species in the Barents Sea for a limited supply of food. It is also likely that new species may enter the Norwegian Sea. |
Fish production patterns are also very likely to be affected, although there are large uncertainties regarding the timing and location of zooplankton and benthic production that serve as prey resources for fish growth, and the wind advection patterns and direction that favor survival of some fish species relative to others. This is an active area of research, presently being addressed by GLOBEC (Global Ocean Ecosystem Dynamics) research programs around the world. Given historical recruitment patterns, it seems likely that herring, cod, and walleye pollock recruitment would be increased under future climate warming scenarios. Benthic-feeding flatfish, such as rock sole in the eastern Bering Sea, would be likely to have higher average recruitment in a warmer Bering Sea. Greenland halibut, capelin, and polar cod would be likely to decline in abundance. The greatest variability in recruitment would occur for all species at the extremes of their ranges.
Migration patterns are very likely to shift, causing changes in arrival times along the migration route. The timing of the spring migration of cod into the Gulf of St. Lawrence appears to be related to the timing of ice melt. In winter, cod appear to congregate at the edge of the sea ice but do not pass beneath it[11]. The spring migration appeared to be delayed by as much as 20 days in 1992, when ice melt was particularly late in the southern region of the Gulf. Change in sea ice distribution is one of the expected effects of climate change that is likely to have pronounced impacts on many fish species. Growth rates are very likely to vary, with the amplitude and direction being species dependent. While cod growth rates in the southern areas of the Arctic are very likely to increase with a rise in water temperature[12], this may not be the case for Arctic Ocean species.
Qualitative predictions of the consequences of climate change on fish resources require good regional atmospheric and oceanic models of the response of the ocean to climate change. Dynamically or statistically downscaled output from global circulation models, which are only recently becoming available, could be very useful. Greater understanding is needed concerning the life histories for those species for which predictions are required, and concerning the role of the environment, species interactions, and fishing in determining the variability of growth, reproduction, distribution, and abundance of fish populations. The multi-forcing and numerous past examples of "failed" predictions of environment–fish relationships indicate the difficulties faced by fisheries scientists in providing reliable predictions of the response to climate change.
Marine mammals and seabirds (9.3.4.5)
The impacts of climate change scenarios on marine mammals and seabirds in the Arctic are likely to be profound, but the precise form these impacts will take is not easy to determine[13]. Patterns of change are non-uniform[14] and highly complex. Oscillations occurring at a variety of scales[15] complicate regional predictions of long-term trends. Also, species responses will vary dramatically[16]. Mesoscale environmental features, e.g., frontal zones and eddies, that are associated with enhanced productivity are important to apex predators, but future changes in these features are not represented well at the present spatial resolution of circulation models[17]. Regional, small-scale coupled air–sea–ice models are needed in order to make reliable projections of change in mesoscale environmental features.
Given the most likely scenarios for changes in oceanographic conditions within the ACIA region by 2020 (Table 9.4), changes in seabird and marine mammal communities are very likely to be within the range(s) observed over the last 100 years. If, however, the increase in water temperature and the sea-ice retreat continue as projected until 2050 and 2080, marine [[ecosystem]s] will change in ways not seen in recent history. One of the first changes expected is a poleward shift in species (and broader assemblages). However, there is a limit to how far north arctic species can shift following the sea ice. Once seasonal sea-ice cover retreats beyond the shelf regions, the oceanographic conditions will change dramatically and become unsuitable for many species. If the loss of sea ice is as dramatic temporally and spatially as has been projected by the ACIA-designated models, negative consequences are very likely within the next few decades for arctic animals that depend on sea ice for breeding or foraging[18]. The worst-case scenarios in terms of reduced sea-ice extent, duration, thickness, and concentration by 2080 are very likely to threaten the existence of whole populations and, depending on their ability to adapt to change, are very likely to result in the extinction of some species. Prospects for long-term abundance projections for populations of large marine predators are not good[19].
Climate change also poses risks to marine mammals and seabirds in the Arctic in terms of increased risk of disease for arctic-adapted vertebrates owing to improved growing conditions for the disease vectors and from introductions via contact with non-indigenous species[20]; increased pollution loads via increased precipitation bringing more river borne pollution northward[21]; increased competition from northward temperate species expansion; and impacts via increased human traffic and development in previously inaccessible, ice-covered areas. Alterations to the density, distribution, or abundance of keystone species at various trophic levels, such as polar bears and polar cod, are very likely to have significant and rapid effects on the structure of the ecosystems they currently occupy.
Although many climate change scenarios focus on negative consequences for [[ecosystem]s], climate change will provide opportunities for some species. The ability to adapt to new climate regimes is often vast, and this potential should not be underestimated; many higher marine vertebrates in the Arctic are adapted to dealing with patchy food resources and high variability in the abundance of food resources.
Marine mammals
Changes in the extent and type of sea ice will affect the distribution and foraging success of polar bears. The earliest impact of warming had been considered most likely to occur at the southern limits of their distribution, such as James and Hudson Bays[22], and this has now been documented[23]. Late sea-ice formation and early breakup means a longer period of annual fasting for polar bears. Reproductive success is strongly linked to their fat stores; females in poor condition have smaller litters and smaller cubs, which are less likely to survive, than females in good condition. There are also concerns that direct mortality rates are likely to increase with the climate change scenarios projected by the ACIA-designated models. For example, increased frequency or intensity of spring rain could cause dens to collapse resulting in the death of the female as well as the cubs. Earlier spring breakup of ice could separate traditional den sites from spring feeding areas, and young cubs forced to swim long distances from breeding areas to feeding areas would probably have a lower survival rate. It is difficult to envisage the survival of polar bears as a species given a zero summer sea-ice scenario. Their only option would be a terrestrial summer lifestyle similar to that of brown bears, from which they evolved. In such a case, competition, risk of hybridization with brown bears and grizzly bears, and increased interactions with people would then number among the threats to polar bears.
Ice-living seals are particularly vulnerable to the changes in the extent and character of arctic sea ice projected by the ACIA-designated models because they depend on the sea ice as a pupping, molting, and resting platform, and some species forage on many ice-associated prey species[24]. Of the high arctic pinnipeds ringed seals are likely to be most affected because many aspects of their life history and distribution are linked to sea ice[25]. They are the only arctic seal species that can create and maintain holes in thick sea ice and hence their distribution extends further north than that of all other pinnipeds. Ringed seals require sufficient snow cover to construct their lairs and the sea ice must be sufficiently stable in spring to rear young successfully[26] (Fig. 9.31). Premature breakup of the sea ice could result in premature separation of mother–pup pairs and hence high neonatal mortality. Ringed seals do not normally haul out on land and to do this would represent a dramatic change in behavior. Land breeding would expose the pups to much higher predation rates, even in a best-case scenario.
Bearded seals use regions of thin, broken sea ice over shallow areas with appropriate benthic prey communities[27]. Their distribution, density, and reproductive success are dependent on the maintenance of suitable sea-ice conditions in these shallow, often coastal, areas. Walruses, another predominantly benthic feeder, also have quite specific sea-ice requirements. They overwinter in areas of pack ice where the ice is sufficiently thin that they can break through and maintain breathing holes[28], but is sufficiently thick to support the weight of groups of these highly gregarious animals. Ice retreat may result in much of the remaining arctic sea ice being located over water that is too deep for these benthic foragers. Also, there is a more general concern that the likely decline in the community of plants, invertebrates, and fishes that live in close association with the underside of sea ice are very likely to result in a dramatic decrease in the flux of carbon to the benthic community, upon which bearded seals, walruses, and other animals such as grey whales depend[29].
Harp seals are flexible about the nature of their summer sea-ice habitat, but during breeding travel to traditional sites in southern waters where they form large herds on extensive areas of pack ice. Massive pup mortality occurs during poor ice years. Hooded seals also breed in traditional areas, but select thicker sea ice than harp seals, and prefer areas where individual floes are large. Females move away from ice edges, presumably to reduce harassment from males[30]. Pup mortality is also high for hooded seals during poor ice years. The situation which occurred during three years in the last two decades when ice did not form in the Canadian Gulf of St. Lawrence breeding area implies severe consequences for harp and hooded seals if spring sea-ice conditions continue to follow current and projected trends. The range and relative abundance of these species is linked to sea-ice cover and climatic conditions[31] and it is not known whether natal site fidelity is maintained for life, regardless of reproductive outcome. Thus, it is difficult to predict whether harp and hooded seals will adjust the location of their breeding and molting activities if spring sea-ice distribution changes dramatically over a relatively short period.
Spotted seals require sea ice over waters of specific depth and so, like bearded seals in the Atlantic, are very likely to be strongly affected by reduced sea-ice extent. The ecological requirements of ribbon seals are so poorly known that the effects of changes in sea-ice conditions are impossible to predict. Their flexibility in shifting from traditional breeding and foraging sites is unknown. Poor seasonal sea-ice conditions will result in a decimation of year-classes in the short term, but in the longer term, herds may form at more northerly sites that meet their needs. Those species that haul out on land when sea ice is not available, such as walrus and spotted seal, may be less affected by changes in sea-ice conditions than the other ice-associated seals.
In contrast, harbour seals and grey seals are likely to expand their distribution in an Arctic with less sea ice. They are for the most part temperate species that have a broad enough niche that they can occupy warm spots in the current Arctic. Other pinnipeds that breed on land in the Arctic are otariid seals. These are likely to be profoundly affected by changes in their food base, as is thought to be happening in the present regime shift in the North Pacific. They could also be affected by heat stress, but Stellar sea lions have a present distribution that includes the Californian coast, implying a considerable tolerance for warm conditions given access to the ocean. Sea otters, like Steller sea lion, have a broad distribution at present and are likely to be most affected by changes at lower trophic levels which affect their food availability.
The impact of climate-induced perturbations on cetaceans is less certain than for ice-breeding pinnipeds and polar bears[32], although Burns[33] suggests grave implications for cetaceans in the Arctic. The uncertainty arises because the link between arctic cetaceans and sea ice is largely via prey availability rather than the sea ice itself[34]. All the northern whales exhibit habitat selection, with sea-ice cover, depth, bathymetric structure, for example, of varying importance[35]. Bowhead whales, beluga whales (Delphinapterus leucas), narwhals, and minke whales can all break young sea ice with their backs in order to breathe in ice-covered areas, but their distribution is generally restricted to areas containing leads or [[polynya]s] and open-water areas at the periphery of the pack-ice zone. Bowhead whales are considered the most ice-adapted cetacean. They feed largely on high arctic copepods and euphausiids[36], and the distribution of these prey species determines their movements and distribution. Bowhead whales have evolved as ice whales, with elevated rostrums (i.e., beaks) and blow holes that allow them to breathe more easily in sea ice; it is not known whether they could adjust to ice-free waters[37]. Bowhead whales are presently an endangered species despite decades of protection from commercial hunting. They consume Calanus spp. and euphausiids and changes in sea-ice conditions are likely to have a major impact on their foraging[38].
Narwhal and beluga are known to forage at ice edges and cracks[39], but are highly migratory and range well south of summer edges in the arctic pack ice[40], foraging along the fronts of glaciers[41] or even in areas of open water[42]. A small, threatened, population of belugas is resident in the Canadian Gulf of St. Lawrence, well south of the Arctic Circle, which has been affected by industrial pollution and habitat disturbance. Tynan and DeMaster[43] predicted that arctic belugas might alter the timing and spatial patterns of seasonal migration given a retreat of the southern ice edge, particularly in the Canadian Archipelago. Vibe[44] reported that the historical beluga distributions are linked to sea ice, wind, and current conditions along the Greenland coast (see section 9.3.3.4 (Future change in processes and impacts on Arctic biota)). The changes projected for arctic sea ice over the coming decades may promote genetic exchange between populations that are currently isolated due to the barrier formed by the southern ice edge. Narwhal utilize coastal habitats in summer, but in winter move offshore to deep-water areas with complex bathymetry. These areas are completely ice-covered except for shifting leads and cracks. Narwhal are thought to feed on cephalopods at this time[45], thus the effects of climate change on narwhal are likely to be via sea-ice distribution patterns and effects on key prey species.
All other cetacean species that frequent the Arctic avoid ice-covered areas. Their distributions are predominantly determined by prey availability[46] and so the impact of climate change will occur indirectly via changes to their potential prey base. Grey whales are unusual in that they are benthic feeders, and so are very likely to be affected by climate change in ways more similar to walruses and bearded seals than other cetaceans.
Seabirds
The effects of climate change on seabird populations, both direct and indirect through effects on the oceans, are likely to be detected first near the limits of the species range and near the margins of their oceanographic range[47]. Brown[48] suggests that the southern limits for many arctic seabird species will move northward, as will their breeding ranges. Changes in patterns of distribution, breeding phenology, and periods of residency in the Arctic are likely to be some of the first responses to climate change observed in arctic seabird populations. This is partly because these are more easily detected than subtle or complex changes such as changes in population size and ecosystem function[49]. Because arctic seabirds are long-lived, have generally low fertility, and live in a highly variable environment, effects of climate change on population size, even if quite significant, may take several years to show[50].
Seabirds are likely to be influenced most by indirect changes in prey availability[51]. Seabirds respond to anything that affects food availability and so are often good indicators of a system’s productivity[52]. Several studies have shown that climate-induced changes in oceanographic conditions can have large-scale and pervasive effects on vertebrate trophic interactions, affecting seabird population size and reproductive success[53]. Species with narrow food or habitat requirements are likely to be the most sensitive[54]. As warmer (or colder) water would affect the distribution of prey, the distribution of individual seabird species is likely to change in accordance with changes in the distribution of macrozooplankton and fish populations. Brown[55] suggests that improved foraging conditions will result in range expansions northward for many species. This is because the retreating pack ice will open up more feeding areas in spring and will provide phytoplankton with earlier exposure to daylight, thereby increasing productivity throughout the Arctic. However, from analyses of probable changes in food availability in sub-antarctic waters, Croxall[56] concluded that it was not possible to be certain whether a change in the amount of sea ice would mean more or less prey for seabirds. Many of these uncertainties are also relevant to arctic areas.
Changes in water temperature are very likely to have significant consequences for pelagic fish species (see Section 9.3.4.4 (Future change in processes and impacts on Arctic biota) and Chapter 13 (Future change in processes and impacts on Arctic biota)). Most fish species are sensitive to changes in water temperature[57], and only slight changes in the thermal regime can induce changes in their temporal and spatial (both vertical and horizontal) distributions[58]. For example, increases in air temperature will probably lead to a greater inflow of warm Atlantic water into the Barents Sea, caused by complex interactions between different water masses, ocean currents, and wind systems in the north Atlantic. This inflow is very likely to displace the Polar Front north- and eastward, especially in the eastern Barents Sea. The ice edge would then be located further north in winter, with a consequent reduction in the phytoplankton bloom which normally follows the receding ice edge during spring and summer. It is likely that the distribution of the Barents Sea capelin would be displaced northeastward, from the central to the northeastern Barents Sea. Important life-cycle changes are likely to include changes in the timing of spawning, with a consequent shift in the timing of migration and a displacement of migration routes[59]. Such changes to capelin alone could have profound consequences for many arctic seabirds in the Barents Sea.
Extreme changes in the spatial and temporal availability of food can have dramatic effects on the survival of adult seabirds[60]. However, seabirds are able to travel great distances and so are insulated to some extent from environmental variability. They are able to exploit locally and ephemerally favorable conditions during much of the year quite freely. However, during the breeding season when they are constrained to return to a land-based breeding site but are dependant on marine resources for foraging, less extreme reductions in prey availability can affect reproductive success. Most Northern Hemisphere seabirds forage within 200 km of their colonies[61]. Because seabirds generally lay only one egg they cannot alter clutch size to compensate for low food availability in a given season. Instead, they reduce the extent of their parental care contribution when resources are in short supply in order to protect their own long-term survival[62]. Because they are long-lived, have delayed sexual maturity, and have conservative reproductive output, even dramatic reductions in fledgling survival may not be apparent in terms of overall population size for several years.
If climate change induces long-term shifts in the spatial distribution of macrozooplankton (predominantly crustaceans) and small schooling pelagic fish, seabird breeding distribution patterns are likely to alter. These prey species are usually concentrated in frontal or upwelling areas, which provide a spatially and temporally predictable food supply for seabirds[63]. If changing environmental conditions cause these oceanographic features to relocate, then prey distributions are very likely to change. If new breeding sites become available in close proximity to the new feeding areas, little change is likely. However, if suitable breeding areas are not available near the relocated fronts or upwelling, the seabirds may not be able to take advantage of available food at its new location during the reproductive season, resulting in reproductive failure. The impacts of future climate change on seabirds are likely to be extremely variable in a spatial context.
Temporal changes in prey availability can also change the timing of breeding in seabirds[64], and potentially result in a mismatch between the timing of reproduction and the time of food abundance[65]. Such a mismatch may have profound impacts on reproductive success[66]. The timing of breeding is especially critical for birds breeding in arctic areas; low temperatures and a restricted period of prey availability create a narrow temporal window in which the nesting period sits[67].
The ivory gull is an exception to many of these general patterns. This species is closely associated with sea ice throughout most of its life cycle. Changes in sea-ice extent and concomitant changes in the distribution of ice-associated seals and polar bears are very likely to result in changes in ivory gull distribution and potentially negative effects on abundance. There is concern that major reductions in ivory gull populations have already occurred[68]. There is also concern that little auks, specialist feeders on arctic copepods during the summer, would be negatively affected by the changes predicted in the "Calanus complex" in the Barents Sea and other parts of the North Atlantic.
Changes in sea level may restrict breeding at existing sites, but may increase the suitability of other sites that are not currently usable owing to, for example, predator access.
Direct evidence of negative effects of environmental conditions (weather) for seabirds is rare, although wind is thought to be important for foraging energetics. Healthy arctic seabirds have little difficulty coping with extreme cold; they are insulated by feathers and subcutaneous fat. However, owing to these adaptations they may have difficulty keeping cool. Warmer [[temperature]s] in the Arctic are very likely therefore to set southern limits to seabird distributions that are unrelated to the availability of prey or breeding sites[69]. Extreme weather can result in direct mortality of chicks or even adults, but it is most likely that the greatest effect of inclement weather would be to restrict the opportunity for seabirds to forage[70]. Heavy rain could flood the nests of burrowing species such as little auks or puffins[71] and freezing rain could affect the thermal balance of exposed chicks leading to mortality[72]. Changes to the normal patterns of wind speed and direction could alter the cost of flight, particularly during migration[73], but it is the nature and extent of the change that determine whether the consequences are negative (or positive) for individual seabird species.
Chapter 9: Marine Systems
9.1. Introduction (Future change in processes and impacts on Arctic biota)
9.2. Physical oceanography
9.2.1. General features (Future change in processes and impacts on Arctic biota)
9.2.2. Sea ice (Sea ice effect on marine systems in the Arctic)
9.2.3. Ocean processes of climatic importance
9.2.4. Variability in hydrographic properties and currents
9.2.5. Anticipated changes in physical conditions
9.3. Biota
9.3.1. General description of the Arctic biota community
9.3.2. Physical factors mediating ecological change
9.3.3. Past variability – interannual to decadal
9.3.4. Future change – processes and impacts on biota
9.4. Effects of changes in ultraviolet radiation
9.5. The carbon cycle and climate change
9.6. Key findings (Future change in processes and impacts on Arctic biota)
9.7. Gaps in knowledge and research needs
References
Citation
Committee, I. (2012). Future change in processes and impacts on Arctic biota. Retrieved from http://editors.eol.org/eoearth/wiki/Future_change_in_processes_and_impacts_on_Arctic_biota- ↑ Hewes, C.D., E. Sakshaug, F.M.H. Reid and O. Holm-Hansen, 1990. Microbial autotrophic and heterotrophic eucaryotes in Antarctic waters: relationships between biomass and chlorophyll, adenosine triphosphate and particulate organic carbon. Marine Ecology Progress Series, 63:27–35.
- ↑ Sakshaug, E. and J.J. Walsh, 2000. Marine biology: Biomass, productivity distributions and their variability in the Barents and Bering Seas. In: M. Nuttall and T.V. Callaghan (eds.). The Arctic: Environment, People, Policy, pp. 163–196. Harwood, Amsterdam.
- ↑ Brodeur, R.D. and D.M. Ware, 1992. Long-term variability in zooplankton biomass in the subarctic Pacific Ocean. Fisheries Oceanography, 1(1):32–38.–Overland, J.E., M.C. Spillane, H.E. Hurlburt and A.J. Wallcraft, 1994. A numerical study of the circulation of the Bering Sea Basin and exchange with the North Pacific Ocean. Journal of Physical Oceanography, 24:736–758.–Skjoldal, H.R., A. Hassel, F. Rey and H. Loeng, 1987. Spring phytoplankton development and zooplankton reproduction in the central Barents Sea in the period 1979–84. In: H. Loeng (ed.). Proceedings of the Third Soviet-Norwegian Symposium, Murmansk, 1986, pp. 59–89. Institute of Marine Research, Bergen, Norway.
- ↑ Brodeur, R.D., H. Sugisaki and G.L. Hunt Jr., 2002. Increases in jellyfish biomass in the Bering Sea: implications for the ecosystem. Marine Ecology Progress Series, 233:89–103.
- ↑ Beaugrand, G., P.C. Reid, F. Ibañez, J.A. Lindley and M. Edwards, 2002. Reorganization of North Atlantic marine copepod diversity and climate. Science, 296:1692–1694.
- ↑ Hansen, B., S. Christiansen and G. Pedersen, 1996. Plankton dynamics in the marginal ice zone of the central Barents Sea during spring: carbon flow and structure of the grazer food chain. Polar Biology, 16:115–218.
- ↑ Thorson, G., 1950. Reproductive and larval ecology of marine bottom invertebrates. Biological Reviews, 25:1–45.
- ↑ Grebmeier, J.M., 1993. Studies of pelagic-benthic coupling extended onto the Soviet continental shelf in the northern Bering and Chukchi Seas. Continental Shelf Research, 13(5–6):653–668.–Grebmeier, J.M., W.O. Smith and R.J. Conover, 1995. Biological processes on Arctic continental shelves: ice-ocean-biotic interactions. In: W.O. Smith and J.M. Grebmeier (eds.). Arctic Oceanography: Marginal Zones and Continental Shelves. Coastal and Estuarine Studies, 49:231–261.
- ↑ Walsh, J.J., C.P. McRoy, L.K. Coachman, J.J. Goering, J.J. Nihoul, T.E. Whitledge, T.H. Blackburn, P.L. Parker, C.D.Wirick, P.G. Stuert, J.M. Grebmeier, A.M. Springer, R.D. Tripp, D.A. Hansell, S. Djenidi, E. Deleersnijder, K. Henriksen, B.A. Lund, P. Andersen, F.E. Muller-Karger and K. Dean, 1989. Carbon and nitrogen cycling within the Bering/Chukchi Seas: Source regions for organic matter effecting AOU demands of the Arctic Ocean. Progress in Oceanography, 22:277–359.
- ↑ Walsh, J.J., C.P. McRoy, L.K. Coachman, J.J. Goering, J.J. Nihoul, T.E. Whitledge, T.H. Blackburn, P.L. Parker, C.D.Wirick, P.G. Stuert, J.M. Grebmeier, A.M. Springer, R.D. Tripp, D.A. Hansell, S. Djenidi, E. Deleersnijder, K. Henriksen, B.A. Lund, P. Andersen, F.E. Muller-Karger and K. Dean, 1989. Carbon and nitrogen cycling within the Bering/Chukchi Seas: Source regions for organic matter effecting AOU demands of the Arctic Ocean. Progress in Oceanography, 22:277–359.
- ↑ Fréchet, A., 1990. Catchability variations of cod in the marginal ice zone. Canadian Journal of Fisheries and Aquatic Sciences, 47(9):1678–1683.
- ↑ Brander, K.M., 1995. The effect of temperature on growth of Atlantic cod (Gadus morhua L.). ICES Journal of Marine Science, 52:1–10.–Michalsen, K., G. Ottersen and O. Nakken, 1998. Growth of North-east Arctic cod (Gadus morhua L.) in relation to ambient temperature. ICES Journal of Marine Science, 55:863–877.
- ↑ Jarvis, P.J., 1993. Environmental changes. In: R.W. Furness and J.J.D. Greenwood (eds.). Birds as Monitors of Environmental Change, pp. 42–77. Chapman & Hall, London.–Shugart, H.H., 1990. Using ecosystem models to assess potential consequences of global climatic change.Trends in Ecology and Evolution, 5:303–307.
- ↑ Parkinson, C.L., 1992. Spatial patterns of increases and decreases in the length of the sea ice season in the north polar region, 1979–1986. Journal of Geophysical Research, 97(C9):14377–14388.
- ↑ Mysak, L.A., D.K. Manak and R.F. Marsden, 1990. Sea-ice anomalies observed in the Greenland and Labrador Seas during 1901–1984 and their relation to an interdecadal Arctic climate cycle. Climate Dynamics, 5:111–133.
- ↑ Kitaysky, A.S. and E.G. Golubova, 2000. Climate change causes contrasting trends in reproductive performance of planktivorous and piscivorous alcids. Journal of Animal Ecology, 69:248–262.
- ↑ Tynan, C.T. and D.P. DeMaster, 1997. Observations and predictions of Arctic climatic change: potential effects on marine mammals. Arctic, 50(4):308–322.
- ↑ Brown, R.G.B., 1991. Marine birds and climatic warming in the northwest Atlantic. In: W.A. Montevecchi and A.J. Gaston (eds.). Studies of High-Latitude Seabirds. 1. Behavioural, Energetic, and Oceanographic Aspects of Seabird Feeding Ecology. Canadian Wildlife Service Occasional Paper 68, pp. 49–54. Environment Canada, Ottawa.–Burns,W.C.G., 2001. From the harpoon to the heat: climate change and the International Whaling Commission in the 21st century. Georgetown International Environmental Law Review, 13:335–359.–Stirling, I. and A.E. Derocher, 1993. Possible impacts of climatic warming on polar bears. Arctic, 46(3):240–245.–Tynan, C.T. and D.P. DeMaster, 1997. Observations and predictions of Arctic climatic change: potential effects on marine mammals. Arctic, 50(4):308–322.
- ↑ Jenkins, M., 2003. Prospects for biodiversity. Science, 302:1175–1177.
- ↑ Harvell, C.D., K. Kim, J.M. Burkholder, R.R. Colwell, P.R. Epstein, D.J. Grimes, E.E. Hofmann, E.K. Lipp, A.D.M.E. Osterhaus, R.M. Overstreet, J.W. Porter, G.W. Smith and G.R. Vasta, 1999. Emerging marine diseases - climate links and anthropogenic factors. Science, 285:1505–1510.
- ↑ Macdonald, R.W., E. Sakshaug and R. Stein, 2003b. The Arctic Ocean: modern status and recent climate change. In: R. Stein and R.W. Macdonald (eds.). The Organic Carbon Cycle in the Arctic Ocean, Chapter 1.2, pp. 6–21. Springer.
- ↑ Stirling, I. and A.E. Derocher, 1993. Possible impacts of climatic warming on polar bears. Arctic, 46(3):240–245.
- ↑ Stirling, I., N.J. Lunn and J. Iacozza, 1999. Long-term trends in the population ecology of polar bears in western Hudson Bay in relation to climate change. Arctic, 52:294–306.
- ↑ DeMaster, D.P. and R. Davis, 1995. Workshop on the Use of Ice-Associated Seals in the Bering and Chukchi Seas as Indicators of Environmental Change. Report of the workshop on Ice-Associated Seals held 29–31 March 1994, National Marine Mammal Laboratory, NOAA, Seattle, 10pp.
- ↑ Finley, K.J., G.W. Miller, R.A. Davis and W.R. Koski, 1983. A distinctive large breeding population of ringed seals (Phoca hispida) inhabiting the Baffin Bay pack ice. Arctic, 36:162–173.–Smith,T.G., M.O. Hammill and G. Taugbøl, 1991. Review of the developmental, behavioural and physiological adaptations of the ringed seal, Phoca hispida, to life in the arctic winter. Arctic, 44:124–131.–Wiig, Ø., A.E. Derocher and S.E. Belikov, 1999. Ringed seal (Phoca hispida) breeding in the drifting pack ice of the Barents Sea. Marine Mammal Science, 15:595–598.
- ↑ Lydersen, C. and K.M. Kovacs, 1999. Behaviour and energetics of ice-breeding, North Atlantic phocid seals during the lactation period. Marine Ecology Progress Series, 187:265–281.
- ↑ Burns, J.J., 1981a. Bearded seal Erignathus barbatus Erxleben, 1777. In: S.H. Ridgway and R.J. Harrison (eds.). Handbook of Marine Mammals,Vol. 2. Seals, pp. 145–170. Academic Press.
- ↑ Stirling, I., H. Cleator and T.G. Smith, 1981. Marine mammals. In: I. Stirling and H. Cleator (eds.). Polynyas in the Canadian Arctic, pp. 45–58. Canadian Wildlife Service Occasional Paper No. 45.
- ↑ Tynan, C.T. and D.P. DeMaster, 1997. Observations and predictions of Arctic climatic change: potential effects on marine mammals. Arctic, 50(4):308–322.
- ↑ Kovacs, K.M., 1990. Mating strategies of male hooded seals (Crystophora cristata). Canadian Journal of Zoology, 68:2499–2505.
- ↑ Vibe, C., 1967. Arctic Animals in Relation to Climate Fluctuations. Danish Zoogeographical Investigations in Greenland. Meddelelser om Gronland, 170(5):227pp.
- ↑ Tynan, C.T. and D.P. DeMaster, 1997. Observations and predictions of Arctic climatic change: potential effects on marine mammals. Arctic, 50(4):308–322.
- ↑ Burns, W.C.G., 2001. From the harpoon to the heat: climate change and the International Whaling Commission in the 21st century. Georgetown International Environmental Law Review, 13:335–359.
- ↑ Moore, S.E., 2000. Variability of cetacean distribution and habitat selection in the Alaskan Arctic, autumn 1982–91. Arctic, 53:448–460.–Moore, S.E., D.P. DeMaster and P.K. Dayton, 2000. Cetacean habitat selection in the Alaskan Arctic during summer and autumn. Arctic, 53:432–447.
- ↑ Moore, S.E., 2000. Variability of cetacean distribution and habitat selection in the Alaskan Arctic, autumn 1982–91. Arctic, 53:448–460.–Moore, S.E., D.P. DeMaster and P.K. Dayton, 2000. Cetacean habitat selection in the Alaskan Arctic during summer and autumn. Arctic, 53:432–447.
- ↑ Lowry, L.F., 1993. Foods and feeding ecology. In: J.J. Burns, J.J. Montague and C.J. Cowles (eds.). The Bowhead Whale, pp. 201–238. Society for Marine Mammalogy Special Publication No. 2. Allen Press, Kansas.
- ↑ Tynan, C.T. and D.P. DeMaster, 1997. Observations and predictions of Arctic climatic change: potential effects on marine mammals. Arctic, 50(4):308–322.
- ↑ Finley, K.J., 2001. Natural history and conservation of the Greenland whale, or bowhead, in the Northwest Atlantic. Arctic, 54:55–76.
- ↑ Bradstreet, M.S.W., 1982. Occurrence, habitat use, and behavior of seabirds, marine mammals, and arctic cod at the Pond Inlet ice edge. Arctic, 35:28–40.–Crawford, R. and J. Jorgenson, 1990. Density distribution of fish in the presence of whales at the Admiralty inlet landfast ice edge. Arctic, 43(3):215–222.
- ↑ Rice, D.W., 1998. Marine Mammals of the World - Systematics and Distribution. Society for Marine Mammalogy Special Publication No. 4. Allen Press, Kansas, 231pp.
- ↑ Lydersen, C., A.R. Martin, K.M. Kovacs and I. Gjertz, 2001. Summer and autumn movements of white whales Delphinapterus leucas in Svalbard, Norway. Marine Ecology Progress Series, 219:265–274.
- ↑ Reeves, R.R., 1990. An overview of the distribution, exploitation and conservation status of belugas, worldwide. In: J. Prescott and M. Gauquelin (eds.). For the Future of the Beluga: Proceedings of the International Forum for the Future of the Beluga, pp. 47–58. University of Quebec Press.
- ↑ Tynan, C.T. and D.P. DeMaster, 1997. Observations and predictions of Arctic climatic change: potential effects on marine mammals. Arctic, 50(4):308–322.
- ↑ Vibe, C., 1967. Arctic Animals in Relation to Climate Fluctuations. Danish Zoogeographical Investigations in Greenland. Meddelelser om Gronland, 170(5):227pp.
- ↑ Dietz, R., M.P. Heide-Jørgensen, P.R. Richard and M. Acquarone, 2001. Summer and fall movements of narwhals (Monodon monoceros) from northeastern Baffin Island towards northern Davis Strait. Arctic, 54:244–261.
- ↑ Ridgway, S.H. and R.J. Harrison, 1981–1999. Handbook of Marine Mammals. Vols. 1–6. Academic Press.
- ↑ Barrett, R.T. and Y.V. Krasnov, 1996. Recent responses to changes in stocks of prey species by seabirds breeding in the southern Barents Sea. ICES Journal of Marine Science, 53:713–722.–Montevecchi, W.A. and R.A. Myers, 1997. Centurial and decadal oceanographic influences on changes in northern gannet populations and diets in the north-west Atlantic: implications for climate change. ICES Journal of Marine Science, 54:608–614.
- ↑ Brown, R.G.B., 1991. Marine birds and climatic warming in the northwest Atlantic. In: W.A. Montevecchi and A.J. Gaston (eds.). Studies of High-Latitude Seabirds. 1. Behavioural, Energetic, and Oceanographic Aspects of Seabird Feeding Ecology. Canadian Wildlife Service Occasional Paper 68, pp. 49–54. Environment Canada, Ottawa.
- ↑ Furness, R.W., J.J.D. Greenwood and P.J. Jarvis, 1993. Can birds be used to monitor the environment? In: R.W. Furness and J.J.D. Greenwood (eds.). Birds as Monitors of Environmental Change, pp. 1–41. Chapman & Hall.–Montevecchi, W.A., 1993. Birds as indicators of change in marine prey stocks. In: R.W. Furness and J.J.D. Greenwood (eds.). Birds as Monitors of Environmental Change, pp. 217–266. Chapman & Hall.
- ↑ Thompson, P.M. and J. Ollason, 2001. Lagged effects of ocean climate change on fulmar population dynamics. Nature, 413:417–420.
- ↑ Brown, R.G.B., 1991. Marine birds and climatic warming in the northwest Atlantic. In: W.A. Montevecchi and A.J. Gaston (eds.). Studies of High-Latitude Seabirds. 1. Behavioural, Energetic, and Oceanographic Aspects of Seabird Feeding Ecology. Canadian Wildlife Service Occasional Paper 68, pp. 49–54. Environment Canada, Ottawa.–IPCC, 1998. The Regional Impacts of Climate Change: An Assessment of Vulnerability. A Special Report of Working Group II of the Intergovernmental Panel on Climate Change. R.T. Watson, M.C. Zinyowera and R.H. Moss (eds.). Cambridge University Press, 527pp.–Schreiber, E.A., 2001. Climate and weather effects on seabirds. In: E.A. Schreiber and J. Burger (eds.). Biology of Marine Birds. CRC Marine Biology Series, Vol. 1, pp. 179–215. CRC Press.
- ↑ Bailey, R.S., R.W. Furness, J.A. Gauld and P.A. Kunzlik, 1991. Recent changes in the population of the sandeel (Ammodytes marinus Raitt) at Shetland in relation to estimates of seabird predation. ICES Marine Science Symposia, 193:209–216.–Hunt, G.L. Jr., J.F. Piatt and K.E. Erikstad, 1991. How do foraging seabirds sample their environment? In: Proceedings of the 20th International Ornithological Congress, pp. 2272–2279. New Zealand Ornithological Congress Trust Board,Wellington, New Zealand.–Montevecchi, W.A., 1993. Birds as indicators of change in marine prey stocks. In: R.W. Furness and J.J.D. Greenwood (eds.). Birds as Monitors of Environmental Change, pp. 217–266. Chapman & Hall.
- ↑ Duffy, D.C., 1990. Seabirds and the 1982–84 El Niño-Southern Oscillation. In: P.W. Glynn (ed.). Global Ecological Consequences of the 1982–83 El Niño-Southern Oscillation, pp. 395–415. Elsevier.–Montevecchi, W.A. and R.A. Myers, 1997. Centurial and decadal oceanographic influences on changes in northern gannet populations and diets in the north-west Atlantic: implications for climate change. ICES Journal of Marine Science, 54:608–614.–Schreiber, R.W. and E.A. Schreiber, 1984. Central Pacific seabirds and the El Niño Southern Oscillation: 1982–1983 perspectives. Science, 225:713–716.
- ↑ Jarvis, P.J., 1993. Environmental changes. In: R.W. Furness and J.J.D. Greenwood (eds.). Birds as Monitors of Environmental Change, pp. 42–77. Chapman & Hall, London.–Vader, W., R.T. Barrett, K.E. Erikstad and K.B. Strann, 1990. Differential responses of common and thick-billed murres to a crash in the capelin stock in the southern Barents Sea. Studies in Avian Biology, 14:175–180.
- ↑ Brown, R.G.B., 1991. Marine birds and climatic warming in the northwest Atlantic. In: W.A. Montevecchi and A.J. Gaston (eds.). Studies of High-Latitude Seabirds. 1. Behavioural, Energetic, and Oceanographic Aspects of Seabird Feeding Ecology. Canadian Wildlife Service Occasional Paper 68, pp. 49–54. Environment Canada, Ottawa.
- ↑ Croxall, J.P., 1992. Southern Ocean environmental changes: effects on seabird, seal and whale populations. Philosophical Transactions of the Royal Society, London, B, 338(1285):319–328.
- ↑ Gjøsæter, H., 1998. The population biology and exploitation of capelin (Mallotus villosus) in the Barents Sea. Sarsia, 83:453–496.
- ↑ Blindheim, J., R. Toresen and H. Loeng, 2001. Fremtidige klimatiske endringer og betydningen for fiskeressursene. Havets miljo 2001. Fisken og Havet, 2:73–78.–Loeng, H., 2001. Klima og fisk – hva vet vi og hva tror vi. Naturen, 125(3):132–140.–Methven, D.A. and J.F. Piatt, 1991. Seasonal abundance and vertical distribution of capelin (Mallotus villosus) in relation to water temperature at a coastal site off eastern Newfoundland. ICES Journal of Marine Science, 48:187–193.–Shackell, N.L., J.E. Carscadden and D.S. Miller, 1994. Migration of pre-spawning capelin (Mallotus villosus) as related to temperature on the northern Grand Bank, Newfoundland. ICES Journal of Marine Science, 51:107–114.
- ↑ Loeng, H., 2001. Klima og fisk – hva vet vi og hva tror vi. Naturen, 125(3):132–140.
- ↑ Baduini, C.L., K.D. Hyrenbach, K.O. Coyle, A. Pinchuk, V. Mendenhall and G.L. Hunt Jr., 2001. Mass mortality of short-tailed shearwaters in the southeastern Bering Sea during summer 1997. Fisheries Oceanography, 10:117–130.–Piatt, J.F. and T.I. van Pelt, 1997. Mass-mortality of Guillemots (Uria aalge) in the Gulf of Alaska in 1993. Marine Pollution Bulletin, 34:656–662.
- ↑ Hunt, G.L. Jr., F. Mehlum, R.W. Russell, D. Irons, M.B. Decker and P.H. Becker, 1999. Physical processes, prey abundance, and the foraging ecology of seabirds. In: N.J. Adams and R. Slotow (eds.). Proceedings of the 22nd International Ornithological Congress, Durban, pp. 2040–2056. BirdLife South Africa, Johannesburg.
- ↑ Øyan, H. and T. Anker-Nilssen, 1996. Allocation of growth in food-stressed Atlantic Puffin chicks. The Auk, 113(4):830–841.–Weimerskirch, H., 2001. Seabird demography and its relationship with the marine environment. In: E.A. Schreiber and J. Burger (eds.). Biology of Marine Birds. CRC Marine Biology Series Volume 1, pp. 115–135. CRC Press.
- ↑ Hunt, G.L. Jr., 1990. The pelagic distribution of marine birds in a heterogeneous environment. Polar Research, 8:43–54.–Hunt, G.L. Jr., F. Mehlum, R.W. Russell, D. Irons, M.B. Decker and P.H. Becker, 1999. Physical processes, prey abundance, and the foraging ecology of seabirds. In: N.J. Adams and R. Slotow (eds.). Proceedings of the 22nd International Ornithological Congress, Durban, pp. 2040–2056. BirdLife South Africa, Johannesburg.–Mehlum, F., G.L. Hunt, M.B. Decker and N. Nordlund, 1998a. Hydrographic features, cetaceans and the foraging of thick-billed murres and other marine birds in the northwestern Barents Sea. Arctic, 51(3):243–252.–Mehlum, F., N. Nordlund and K. Isaksen, 1998b. The importance of the "Polar Front" as a foraging habitat for guillemots Uria spp. breeding at Bjørnøya., Barents Sea. Journal of Marine Systems, 14:27–43.–Schneider, D.C., 1990. Seabirds and fronts: a brief overview. Polar Research, 8:17–21.–Watanuki, Y., F. Mehlum and A. Takahashi, 2001. Water temperature sampling by foraging Brunnich's Guillemots with bird-borne data loggers. Journal of Avian Biology, 32:189–193.
- ↑ Schreiber, E.A., 2001. Climate and weather effects on seabirds. In: E.A. Schreiber and J. Burger (eds.). Biology of Marine Birds. CRC Marine Biology Series, Vol. 1, pp. 179–215. CRC Press.
- ↑ Visser, M.E., A.J. van Noordwijk, J.M. Tinbergen and C.M. Lessells, 1998. Warmer springs lead to mistimed reproduction in great tits (Parus major). Proceedings of the Royal Society of London: Biological Sciences, 265:1867–1870.
- ↑ Brinkhof, M.W.G., A.J. Cavé and A.C. Perdeck, 1997. The seasonal decline in the first-year survival of juvenile coots: an experimental approach. Journal of Animal Ecology, 66:73–82.
- ↑ Hamer, K.C., E.A. Schreiber and J. Burger, 2001. Breeding biology, life histories, and life history–environment interactions in seabirds. In: E.A. Schreiber and J. Burger (eds.). Biology of Marine Birds. CRC Marine Biology Series, 1:215–259. CRC Press.
- ↑ Krajick, K., 2001. Arctic life, on thin ice. Science, 291:424–425.–Mallory, M.L., H.G. Gilchrist, A.J. Fontaine and J.A. Akearok, 2003. Local ecological knowledge of ivory gull declines in Arctic Canada. Arctic, 56(3):293–298.
- ↑ Brown, R.G.B., 1991. Marine birds and climatic warming in the northwest Atlantic. In: W.A. Montevecchi and A.J. Gaston (eds.). Studies of High-Latitude Seabirds. 1. Behavioural, Energetic, and Oceanographic Aspects of Seabird Feeding Ecology. Canadian Wildlife Service Occasional Paper 68, pp. 49–54. Environment Canada, Ottawa.
- ↑ Harris, M. and S. Wanless, 1984. The effect of the wreck of seabirds in February 1983 on auk populations on the Isle of May (Fife). Bird Study, 31:103–110.
- ↑ Rodway, M.S., J.W. Chardine and W.A. Montevecchi, 1998. Intra-colony variation in breeding performance of Atlantic puffins. Colonial Waterbirds, 21(2):171–184.–Schreiber, E.A., 2001. Climate and weather effects on seabirds. In: E.A. Schreiber and J. Burger (eds.). Biology of Marine Birds. CRC Marine Biology Series, Vol. 1, pp. 179–215. CRC Press.
- ↑ Burger, J. and M. Gochfeld, 1990. The Black Skimmer: Social Dynamics of a Colonial Species. Columbia University Press, New York. 355pp.
- ↑ Furness, R.W. and D.M. Bryant, 1996. Effect of wind on field metabolic rates of breeding Northern Fulmars. Ecology, 77(4):1181–1188.–Gabrielsen, G.W., F. Mehlum and K.A. Nagy, 1987. Daily energy expenditure and energy utilization of free-ranging black-legged kittiwakes. Condor, 89:126–132.