Global marine biodiversity trends
Contents
- 1 Introduction Although marine species richness (Global marine biodiversity trends) may only total 4% of global diversity, life began in the sea, and much of the diversity in the deep branches of life's tree is still primarily or exclusively marine. For example, 35 animal phyla are found in the sea, 14 of which are exclusively marine, whereas only 11 are terrestrial and only one exclusively so. Our understanding of major changes in marine diversity over deep time is comparatively good, thanks to the excellent fossil record left by many marine organisms, although considerable sampling problems limit the potential for accurate fine grained analyses. In contrast, our knowledge of marine diversity in the present is poor compared to our knowledge for terrestrial organisms, and an appreciation for the dramatic changes in marine ecosystems that have occurred in historic times is only just beginning to emerge.
- 2 What is marine biodiversity?
- 3 Estimating marine biodiversity today
- 4 Temporal patterns in marine biodiversity
- 5 Recent and current marine biodiversity trends and drivers
- 6 Consequences of biodiversity loss: ecosystem function and services
- 7 The future of marine biodiversity: the unknown and the unknowable
- 8 Further Reading
Introduction Although marine species richness (Global marine biodiversity trends) may only total 4% of global diversity, life began in the sea, and much of the diversity in the deep branches of life's tree is still primarily or exclusively marine. For example, 35 animal phyla are found in the sea, 14 of which are exclusively marine, whereas only 11 are terrestrial and only one exclusively so. Our understanding of major changes in marine diversity over deep time is comparatively good, thanks to the excellent fossil record left by many marine organisms, although considerable sampling problems limit the potential for accurate fine grained analyses. In contrast, our knowledge of marine diversity in the present is poor compared to our knowledge for terrestrial organisms, and an appreciation for the dramatic changes in marine ecosystems that have occurred in historic times is only just beginning to emerge.
What then can we say about recent trends in the state of marine biodiversity and what they imply for its future? How have and will these changes in marine biodiversity affect the provision of essential ecosystem services (Marine ecosystem services)? In this review, we synthesize the current state of knowledge on global marine biodiversity, discussing composition and function, as well as patterns across time, space and levels of complexity ranging from populations to ecosystems. Our specific goals are to (a) define marine biodiversity, (b) describe the historic trends in biodiversity unrelated to human activities, (c) review recent biodiversity trends and the role of human drivers, (d) assess the functional consequences of recent and future change, and (e) synthesize the unknowns and the unknowables of marine biodiversity and suggest priorities for marine biodiversity research and conservation.
What is marine biodiversity?
 Vibrant coral reefs harbor diverse communities of life in the tropical oceans. Like trees, corals produce annual rings that store a record of past conditions. Chemical analyses reveal details about past temperature, nutrient availability, salinity, and other information. (Photograph courtesy NOAA Photo Library)
Vibrant coral reefs harbor diverse communities of life in the tropical oceans. Like trees, corals produce annual rings that store a record of past conditions. Chemical analyses reveal details about past temperature, nutrient availability, salinity, and other information. (Photograph courtesy NOAA Photo Library) Marine biodiversity is the variety of life in the sea, encompassing variation at levels of complexity from within species to across ecosystems. Biodiversity is not a simple concept like temperature or volume but rather multidimensional. It can thus be measured in different and complementary ways and have different units. Any single measure of diversity (so-called inventory diversity) has four conceptual components: the numbers of entities (or compositional diversity, the most common measure being species richness), the distribution of abundances of these entities in communities structural diversity, the most common measures being evenness equitability and ecodiversity (which combines evenness and richness), the degree to which the entities differ (e.g., divergence when measured genetically, disparity when measured morphologically), and the functional role (trophic, metabolic, habitat forming) these entities play in ecosystems (Table 1).
The complexity of units and scale makes it impossible to assess the state of marine biodiversity using a single measure. Most studies dealing with biodiversity patterns report the simplest measure of biodiversity, that is, species richness. Although species richness may be useful for comparison of taxonomic diversity between ecosystems or within ecosystems over time, it may not give us a good measure of the structure or function of these ecosystems. Moreover, different measures can suggest different conclusions. For example, areas with low ?-diversity can have high ?-diversity; thus it may be risky to use single measures for management or conservation purposes. Similarly, patterns of species diversity and diversity measured at higher taxonomic levels are not always concordant. To obtain a measure of the current state and the dynamics of ecosystems, data on evenness of species abundance or functional measures of biodiversity are usually more appropriate.
Estimating marine biodiversity today
| Table 1. Dimensions and measures of marine biodiversity | |||
| Scale | Compositional | Structural | Funtional |
| Species/ populations | Within-species genomic diversity, divergence, disparity | Abundance | Within-species gene expression and divergence |
| Communities/ ecosystems | ?-diversity, ?-diversity | Ecodiversity, evenness, disparity, ecodiversity spectra (?-diversity), food web complexity | Functional diversity |
| Regional to global | ?-diversity, community/ecosystem diversity | Ecodiversity spectra (?-diversity) | Functional diversity |
There are approximately 300,000 described marine species, which represent about 15% of all described species. There is no single listing of these species, but any such listing would be only an approximation owing to uncertainty from several sources. As a consequence, the total number of marine species is not known to even an order of magnitude, with estimates ranging from 178,000 species to more than 10 million species. The two biggest repositories of marine biodiversity are coral reefs (because of the high number of species per unit area) and the deep sea (because of its enormous area). Estimates for coral reefs range from 1 to 9 million species, but they are very indirect as they are based on a partial count of organisms in a large tropical aquarium or on extrapolations stemming from terrestrial diversity estimates. Estimates for the deep sea are calculated using actual field samples, but extrapolations to global estimates are highly controversial. The largest estimate (10 million benthic species) was based on an extrapolation of benthic macrofauna collected in 233 box cores (30 × 30 cm each) from fourteen stations, although others suggested 5 million species as a more appropriate number. Briggs argued that these enormous figures are excessive extrapolations from small-scale samples, and May suggested instead a total of 500,000 living marine species.
What is clear from these debates is that we have a remarkably poor grasp of what lives in the ocean today, although ongoing programs such as the Census of Marine Life should yield greatly improved estimates in the not too distant future. However, intensive surveys of individual groups point to the enormous scale of the task ahead.
One can, however, make progress in understanding marine diversity through comparisons of different regions because robust differences can potentially be documented in the absence of complete counts. The spatial patterns of global marine biodiversity, including species richness and endemicity, have been subject to excellent reviews. Primary findings include well-documented gradients with respect to latitude (higher diversity in the tropical waters as has been found on land), longitude (decreasing diversity as one moves west to east in the tropical Pacific and Atlantic), and depth. However, there are some disagreements about the reality of some patterns and enormous disagreement about the underlying causes of the patterns. High levels of endemicity are associated with isolated islands, although again there is disagreement and the data are limited to a few well-known taxa.
These marine estimates, inexact as they are, account only for multicellular Eucarya and do not include single-celled eukaryotes, Bacteria, Archaea, and viruses (Marine viruses). Microbial species richness has not been properly quantified at global scales, but recent studies suggest that microbial diversity may be enormous. These studies suggest that even the most conservative extrapolation from small samples may yield global microbial species richness estimates on the order of millions.
Our knowledge of diversity at the community level at local and regional scales is relatively poor. Many coastal regions lack even a simple description of the zonation of shallow benthic communities, and only a limited number of regions have data on ?- or ?-diversity. However, conservation efforts have prompted some excellent community and habitat mapping at regional scales, such as in the Great Barrier Reef in Australia.
The gaps in knowledge of community diversity are even greater at the global scale. There have been no integrated global efforts to count and map the number of distinct ecological communities similar to those carried out for terrestrial ecosystems. The closest attempt is the Large Marine Ecosystems (LME) project. LMEs are 64 nearshore regions characterized by depth, hydrography, productivity, and trophically dependent populations. Although LMEs may be useful for management of exclusive economic zones at regional scales, they do not provide much insight into biodiversity (Marine biodiversity) at the community level. The proposed LMEs encompass huge areas (on the order of hundreds of thousands km2), and a single LME, such as the California current, can harbor ecosystems ranging from cold temperate to subtropical. Additional work has characterized large ocean floor and open ocean regions on the basis of depth, topography, temperature, and productivity. The ecoregions obtained using those methods are also large, and because they are based mostly on physicochemical parameters (which are easier to measure at large scales than biological parameters), they do not provide a detailed picture of biological distinctness.
Temporal patterns in marine biodiversity
Because marine biodiversity is a dynamic entity and we are interested in human impacts, static diversity estimates are less useful than an understanding of trends. Thus, instead of simply asking what is the current state of marine biodiversity, we should ask: What are the trends in marine biodiversity and are current biodiversity trends different from historical trends? To answer these questions, we need to use a historical perspective and compare rates of change across evolutionary and ecological timescales in the absence of human disturbance. The former provides a broad sense of the extremes of changes in planetary marine diversity against which human impacts can be scaled; the latter is more relevant for understanding the role of humans in recent biodiversity change, for making real-time biodiversity assessments, and for applying biodiversity science to management.
Biodiversity change over evolutionary timescales
The number of marine taxa, particularly large complex forms, increased dramatically with the onset of the Cambrian explosion about 540 Million years ago. Sepkoski’s classic work documented a steady increase in the number of taxa during the Phanerozoic, with the exception of five big events during which diversity suffered mass depletion. The events at the end of the Ordovician, Permian, and Cretaceous periods were due to only mass extinctions, whereas the loss in diversity in the late Devonian and at the end of the Triassic was a result of low origination as well as high extinction. However, this paradigm of monotonic increase broken only by mass extinction events has been recently questioned because of sampling artifacts associated with the fossil record, and some authors suggest that during some geological periods taxonomic diversity might have remained stable.
Ecosystems have also changed over geological time, with feedbacks that have changed Earth’s physical properties (e.g., creation of the present atmosphere). Although the information on ecosystem diversity over geological times is not as good as that on taxonomic diversity, it is clear that the number of marine ecosystems and ways of making a living has increased since the primordial pre-Cambrian ocean. Examples include the marine Mesozoic revolution (MMR) that followed the end-Permian mass extinction. During the MMR, there was a proliferation of new plant and animal taxa associated with an increase in trophic diversity, from infaunal suspension and detritus feeders (animals that live in the sediment and filter the water (Seawater) or eat detritus on the bottom) to nektonic carnivores (animals that swim and eat invertebrates and fish in the water column).
Understanding mass extinctions is of particular importance because some have argued that the impact of humans could potentially approach the scale of that caused by asteroids. We clearly have yet to approach the 98% species extinction level that occurred at the end of the Permian, but this should not be used to justify complacency, as threshold effects could result in rapid collapses with little warning. Extinction events associated with global warming are potentially very informative with respect to understanding how marine organisms might respond to a warmer world.
Biodiversity change over ecological timescales
Marine biodiversity naturally changes locally at scales of years to centuries in what has been called ecological succession. A major successional sequence typically begins with some kind of disturbance that either creates new habitat (e.g., a lava flow or a whale fall) or removes habitat-creating dominants (e.g., a storm). The ensuing biotic changes that occur in the absence of human impacts show regularities that can help us understand biodiversity trends caused by human drivers. During a natural successional sequence and in the absence of further disturbance, biodiversity tends to slowly increase over time in a self-organization process that is a consequence of the activities of the organisms themselves. For example, species richness, evenness, and functional diversity generally increase in a nonlinear trend during much of a successional sequence. However, evenness of individual assemblages may saturate or decline during late (climatic) successional stages when ephemeral species disappear, competition for space is strong, and a few species dominate (e.g., algae in kelp forests or corals in shallow coral reefs). The resulting decline can be described in the context of the intermediate disturbance hypothesis: Diversity is lower at high disturbance levels when few opportunist species prevail and at low disturbance levels when a few long-lived, competitively dominant species monopolize the community biomass. When a disturbance occurs in a mature system with high biodiversity, it may decrease biodiversity by eliminating species, or it may cause a competitive release and enhance evenness if the system is dominated by a few foundation species. Small-scale disturbances also enhance diversity at the landscape scale by creating a mosaic of patches in different successional states.
It is worth noting, however, that different measures of biodiversity do not necessarily show parallel trends. Also, because most relevant studies do not include complete censuses but rather just single assemblages or the ecological dominants, it remains unclear whether evenness of the entire community usually declines in late successional stages. Despite these uncertainties, it is clear that disturbance is generally followed by recovery in the direction of late climatic successional stages that predictably become established late in succession when human impacts are lacking or minimal. Below, we summarize some of the best-studied cases.
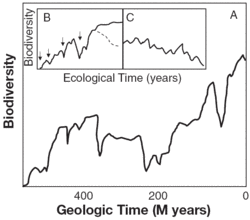 Figure 1. General trends in marine biodiversity over evolutionary and ecological times. (A) General increase over geological timescales, punctuated by declines caused by mass extinctions. Abbreviation: M, million. (B) Solid line: typical trend of marine biodiversity (e.g., species richness, ecodiversity, evenness, functional diversity) over ecological timescales in the absence of human disturbance. Arrows indicate pulse disturbances that reset succession. Dashed line represents decrease in ecodiversity during late successional stages in communities with competitively dominant (architectural) species. (C) Marine biodiversity trends under chronic human disturbance.
Figure 1. General trends in marine biodiversity over evolutionary and ecological times. (A) General increase over geological timescales, punctuated by declines caused by mass extinctions. Abbreviation: M, million. (B) Solid line: typical trend of marine biodiversity (e.g., species richness, ecodiversity, evenness, functional diversity) over ecological timescales in the absence of human disturbance. Arrows indicate pulse disturbances that reset succession. Dashed line represents decrease in ecodiversity during late successional stages in communities with competitively dominant (architectural) species. (C) Marine biodiversity trends under chronic human disturbance. In Mediterranean rocky bottom algal assemblages, species richness increases during succession, but ecodiversity decreases at the end of the annual succession. The successional end point of these algal assemblages is domination by canopy species that monopolize the biomass, although they provide a high degree of structure and microhabitats that result in greater species richness and functional diversity. Similarly, kelp forests in California exhibit a recurrent increase in biomass and vertical complexity after periodic disturbances in the form of storms or El Niño events and exhibit a mosaic of patches of varying diversity (at different successional stages). The colonization of bare substrate results in an increase of algal species richness, ecodiversity, and evenness until a peak is reached months later. Afterward, species richness and ecodiversity may decline slightly because ephemeral early successional species become rare and competitive dominant kelps monopolize the biomass.
Coral reefs are subject to periodic disturbances from a variety of sources, and until relatively recently, reef recovery was the norm. For example, multidecadal monitoring studies have shown that, after a hurricane damages a reef (reducing its coral cover) and in the absence of other disturbances, there is a predictable trend of recovery. Grigg & Maragos studied the recolonization of lava flows by corals in Hawaii and showed that the number of species increases over time. They also found that ecodiversity also increases over time, although it declines slightly before the successional end point is reached. The generality of these patterns in the absence of human disturbance is also supported by analyses of the fossil record.
The level of disturbance has implications for biodiversity (Marine biodiversity) across spatial scales. Between ecosystems, we would expect greater biodiversity in low-nutrient or -energy systems, whereas the likelihood of monocultures or dominance of a few architectural species is greater in systems subject to high-nutrient or -energy inputs. Within ecosystems, high-energy [[habitat]s] would also have less biodiversity than low-energy habitats. For example, Caribbean coral reef benthic communities were dominated by single species of Acropora (a coral with high growth rates) in shallow habitats subject to strong wave energy, whereas in deeper, calmer habitats coral abundance was shared more evenly among many coral species.
These relationships remind us that comparisons of biodiversity between communities may not be appropriate. More biodiversity does not necessarily mean a healthier community; site-specific biodiversity depends on the local upper limits of biodiversity and the constraints imposed by external energy inputs. Moreover, the largest differences among regions in biodiversity appear to be driven more by the regional species pool than by local conditions. In summary, community self-organization (succession) and disturbance interact to create nonlinear relationships where (a) biodiversity within a community tends to increase until the community reaches mature successional stages, (b) biodiversity is higher in habitats or patches subject to intermediate disturbance levels, and (c) the effects of disturbance depend on the level of the disturbance and the predisturbance biodiversity. There is hence a general trend of biodiversity change and return toward predisturbance stages that are recurrent in systems subject to pulse disturbances (Figure 1). As we will see below, chronic disturbances such as those associated with human activities disrupt this process and inhibit the accretion of biodiversity.
Recent and current marine biodiversity trends and drivers
Before humans began to significantly exploit the ocean, the only disturbances resetting the successional clock and causing sudden declines in biodiversity at all levels were environmental disturbances of the type outlined above. However, human activities are without doubt now the strongest driver of change in marine biodiversity at all levels of organization; hence, future trends will depend largely on human-related threats. What is the current marine biodiversity trend? What has been the trend since the beginning of significant human activity in the sea? Because threats typically act in synergy and can produce complex patterns of change, here we focus on the types of biodiversity change that are occurring across scales ranging from species to ecosystems, rather than treating each threat separately.
Species/population trends
The earliest and most conspicuous change in marine biodiversity due to human activities affects the abundance of individual species. The most common changes range from population reductions to global extinction caused by overexploitation or habitat loss, although humans can also increase the abundance of some species through biological introductions.
Global Extinctions
Humans have directly caused the global extinction of more than 20 described marine species, including seabirds, marine mammals, fishes, invertebrates, and algae. Probably the most dramatic example of human-driven extinction in the sea was the Steller’s sea cow (Hydrodamalis gigas), a huge herbivore of the nearshore northeast Pacific that was hunted to extinction within only 27 years of its discovery by Europeans. Another example of rapid hunting-related extinction of a species inhabiting a large ecosystem is the Caribbean monk seal (Monachus tropicalis), which was heavily hunted by Europeans beginning in 1492 and last seen in 1952. However, not all extinctions are caused by overharvesting; for example, the eelgrass limpet Lottia alveus disappeared following the catastrophic decline of its required eelgrass (Zostera marina) habitat because of disease in the northwest Atlantic in the early 1930s.
Many species may have disappeared unnoticed. Losses of species that have not been described are difficult to estimate, but many small species with localized dispersal and limited geographic ranges have already probably gone extinct. Statistical methods can be used to make estimates of loss rates, much as they have been used for tropical rainforests. Assuming that we have already lost 5% of coral reef area, and using an area-species richness power law, it has been estimated that about 1% of coral reef species have already become extinct. Other unnoticed extinctions have undoubtedly occurred in [[habitat]s] that are less known, such as in the deep sea. Seamounts, for example, harbor huge species richness and high levels of endemicity (from 30% to 50% of endemic invertebrates per seamount). Seamount biodiversity is threatened by large-scale commercial trawling, and repeated fishing of a single seamount could mean a large number of species extinctions. The diversity (Marine biodiversity) associated with deep-sea coral reefs is similarly threatened.
Local, regional, and ecological extinctions
Documented local and regional extinctions are more widespread. For example, the gray whale (Eschrichtius robustus) disappeared from the Atlantic in the seventeenth century because of overhunting. Similarly, 9 of 14 species of canopy-forming algae have disappeared since the 1930s from the rocky shores of the northwest Mediterranean; 7 were brown algae from the genus Cystoseira, which were previously the dominant species from the surface to 50 m depth. These algal extinctions were caused by a combination of overgrazing by sea urchins, chemical pollution, habitat destruction, increase in water turbidity, and direct plant removal by net fishing.
.jpg) Gray whale (Eschrichtius robustus). (Source: NOAA)
Gray whale (Eschrichtius robustus). (Source: NOAA) Global or regional losses of species are only the last steps of marine biodiversity decreases. Ecological (or functional) extinction, which occurs long before species completely disappear, is an early sign, and there are many such examples in the sea. Ecological extinction occurs when a species is so rare that it no longer fulfills its natural ecosystem function. That is, the species becomes ecologically irrelevant, with potentially dramatic impacts on biodiversity at higher organizational levels as discussed below. It is difficult to determine when a species becomes ecologically extinct, and we are only now realizing the extent to which populations have declined owing to human activities. Nevertheless, we can assume that species in the IUCN Red List of Threatened Species are ecologically extinct (there are 761 marine species listed). Species that become commercially extinct (no longer profitably harvested) are also likely to be ecologically extinct.
An example of ecological extinction is the vaquita (Phocoena sinus), a small porpoise that lives in the upper Gulf of California whose abundance has been reduced to just a few hundred and is still decreasing. Elasmobranchs (sharks and rays) are particularly vulnerable to ecological extinction because they are heavily targeted, they are caught inadvertently as bycatch in trawling and longline fisheries, and have low reproductive rates. For instance, 14 species of elasmobranchs have virtually disappeared from the northwest Mediterranean since 1957, and 9 species of elasmobranchs have disappeared from the Bay of Biscay since 1727.
The estimation of the potential number of ecological extinctions caused by overfishing is made easier because these extinctions are typically nonrandom. Fishing preferentially targets large species or species high in the food web and sequentially shifts to smaller species, typically in lower trophic levels once the former are no longer commercially profitable, in what has been called “fishing down the food web”. Estimates suggest that large predatory fishes in the ocean have seen their abundance reduced to 10% of historical abundance in the last 50 years, and in the case of sensitive species, such as sharks, to about 1% of their carrying capacity. Tuna and billfish diversity, understood as the number of species caught in longline sets, has declined between 10% and 50% in all oceans. On coral reefs, the historical pattern of decline started with the loss of large [[herbivore]s (Herbivory)] such as sea cows and green turtles, followed by large carnivores such as seals and large fishes, followed by smaller mobile animals. This pattern is general and global and has consequences for the organization of entire communities. The exception to the rule is the nonselective effect of trawling and dredging on marine biodiversity, with depletions of species belonging to all trophic levels. Such indiscriminate impacts are particularly catastrophic in the deep sea, where reproductive rates are often low.
Population declines
Population declines precede ecological extinction and are even more pervasive in the sea. The single most important global indicator of population depletion is the global wild fisheries catch, which, contrary to previous notions, has been declining since the 1990s. In the United States alone, 81 out of 304 exploited stocks for which the status is known are overfished, 93 are either overfished or experiencing overfishing, and 65 are experiencing overfishing.
Disturbances that are not direct extractive activities, such as global warming, may affect a larger number of species than targeted exploitation in a short period of time and at small spatial scales. In this regard, human impacts on water quality (toxic pollutants, nutrients, carbon, acidity) and temperature are the most important and may have differential impacts on particular species guilds or regions. For example, a warming event in 1999 caused massive mortalities of at least 16 species of benthic suspension feeders in the rocky shores of the northwestern Mediterranean. A classic example of massive guild decline is coral bleaching. Elevated seawater temperatures (sometimes associated with extreme El Niño events) result in the loss of symbiotic zooxanthellae and eventually coral death. Bleaching has been more frequent and extreme in the past decades; in 1998, for example, about 80% of the corals in the Indian Ocean bleached and 20% died.
It has been suggested that global warming will also shrink the distribution of polar species and colder water species that cannot migrate poleward. In the Hudson and James Bays of Canada, sea ice now melts earlier in the spring and forms later in the fall. The availability of ice to polar bears is thus shorter, which causes a decline in overall body condition and in reproductive success. Some bodies of water, such as the Gulf of California and the Mediterranean, have no easy exit points for colder water species to use as migration corridors. In the long run, these habitat reductions could lead to local extinctions or global extinctions when the entire range of a species is affected.
More complex responses to global warming are also anticipated. Helmuth et al. suggested that in the intertidal, global warming and the timing of low tides creates a mosaic of thermal environments that may cause a series of localized extinctions in the intertidal at a series of hot spots. Changes in seawater temperature driven by climate variability have also been linked to fish recruitment and habitat shifts (such as in the case of cod, Gadus morhua, off Labrador and Newfoundland); hence, global warming is likely to affect marine biodiversity at the species level in multiple ways. When combined with other disturbances such as overfishing, the effects of global warming might be more pervasive and unpredictable than previously thought.
Links between global warming and disease will increase the severity of threats to biodiversity associated with climate change. There is a recent pattern of increased incidence of disease in marine species belonging to a large number of taxa from sea turtles to sea urchins. Disease can contribute to population declines; generalist pathogens that infect multiple host species and specialist pathogens that attack strong interactors or ecological dominants have particularly dramatic effects.
Other aspects of water quality are also of concern. Eutrophication and pollution also cause declines in species abundance and increase of microbial communities. For example, human waste has been shown to enhance coral disease via the growth of bacterial or fungal populations. In extreme cases enormous dead zones can result. The biological impacts of ocean acidification are largely undocumented but potentially severe for corals or other organisms that build calcium carbonate skeletons.
Population increases and species invasions
Not all species decline in abundance because of human activities. Seagulls, for instance, have adapted to the production of waste from fisheries or in landfills and expanded their distribution and abundance following human settlement. Highly invasive species can colonize new regions and eventually form monocultures. Although the arrival of new species may be seen as an increase in species richness, the consequences for the local biodiversity are generally negative, sometimes catastrophically so.
 The green alga Caulerpa taxifolia clone invading sea grass bed in the Mediterranean. (Source: Marine Conservation Biology Institute)
The green alga Caulerpa taxifolia clone invading sea grass bed in the Mediterranean. (Source: Marine Conservation Biology Institute) The Mediterranean Sea is a classic example of biological invasions, with over 85 species of introduced macrophytes, some of which have become invaders. The most notorious case concerns the introduction and invasion of the tropical green alga Caulerpa taxifolia. This alga was found for the first time in 1984 when it formed a 1-m2 patch in front of the Monaco Aquarium, and it occupies now more than 30,000 ha throughout the Mediterranean, forming monocultures where previously there were complex algal assemblages. Caulerpa racemosa, another tropical alga, began invading the Mediterranean in the 1990s and is increasing at a faster rate than C. taxifolia. These are two extreme examples of rapid invasions associated with massive biodiversity (Marine biodiversity) loss.
Many introductions occur insidiously over long distances, via the ballast water and hulls of ships or on marine debris. Anthropogenic debris is long lasting and slow moving; it has doubled the propagation of fauna in the subtropics and more than tripled it at high (>50°) latitudes. Ballast water in ships is probably the most important introduction vector of both marine and freshwater species into coastal areas. It is estimated that as many as 3000 alien species are transported daily in ballast water, although only a few of them survive the trip and/or establish themselves in a new environment. Estuaries have been particularly impacted; San Francisco Bay, for example, had more than 230 introduced species in 1998, and a significant proportion of these species come via ballast water.
Human activities are also promoting the arrival of new species simply by changing environmental conditions. Global warming has already increased the distributions of warm-water species poleward and thus increased their numbers in locations where they were previously rare or absent. In some cases, this may be expected to increase biodiversity (Marine biodiversity), given the strong latitudinal gradient of biodiversity. However, global warming may also facilitate the successful establishment of destructively invasive species.
Evolutionary consequences
What do these dramatic changes in abundance mean for genetic diversity and evolutionary dynamics? Fishing, habitat destruction, pollution, and other human activities can deplete populations to such a level that most genetic variability is lost. This is typical of relict populations of endangered animals such as the white abalone in southern California. In addition, population declines also cause directional evolutionary changes especially because fishing typically targets the larger individuals and thus selects for individuals that grow slower and reproduce earlier and at smaller sizes (with consequently decreased reproductive output). Some of these changes in genetic diversity could inhibit the ability of the species to recover, and the larger the decline the more pervasive the genetic loss.
By the same token, chronic pressure on ever-declining populations is probably inhibiting evolution and speciation of large vertebrates, causing a decline in biodisparity (the biota’s manifest morphological and physiological variety), and it may result in inbreeding or breakdown of species boundaries as potential mates become scarce. Evolutionary impacts are particularly worrying for large marine species with low population turnover, such as sharks and marine mammals. However, human activities might enhance the evolution of microbes.
Community/ecosystem trends
The extinction of individual species can have ecosystem-wide impacts on biodiversity (Marine biodiversity), although not all species have functional roles of similar importance. For instance, loss of a few predator species often has impacts comparable in magnitude to those caused by a large reduction in plant diversity. To understand the effects on biodiversity caused by the removal of single species, we need to consider the nature and strength of the role of these species in the community. These roles can be predatory, facilitating, or habitat forming.
Changes in tropic relationships: top-down effects
Strong interactors are species capable of preventing the development of a monoculture or of destroying one already established. In other words, these are species whose removal causes explosive increases in the abundance of their (competitively dominant) prey. Sala & Graham showed that per capita interaction strength increases with the size of the predator until it saturates. Because of the nonrandom global pattern of initial depletion of large species, we can expect that fishing removes strong interactors first, with subsequent global food web changes. If the species that are consumed by strong predators have an important community role, such as a habitat-forming species or a major consumer of primary producers, the community-wide changes are the most dramatic and may involve shifts in the structure of entire ecosystems.
The removal of predators can reduce species richness and biomass by orders of magnitude and cause a decline in structural diversity. The classic example is the desertification of algal-dominated communities via sea urchin grazing after the depletion of sea urchin predators. The removal of sea otters in Alaska resulted in the overgrazing of the kelp forests by sea urchins. In the Mediterranean rocky infralittoral, sea urchins in the absence of their normal predators can overgraze complex algal assemblages and shift the community to a barren state, resulting in a species richness and biomass decline of more than one order of magnitude. A similar case occurred in New Zealand kelp forests, where the removal of predatory fishes provoked the formation of sea urchin barrens and a dramatic reduction of benthic primary production. In Kenyan coral reefs, the removal of triggerfish caused a reduction of coral cover under extreme sea urchin densities. In the northeast Pacific, Paine manipulated the abundance of intertidal grazers and showed that consumers control the production of algal assemblages. When predators of grazers were present, the system reached a peak in primary productivity (an average of 86 kg of wet mass m?2 year?1), whereas in the absence of predators, production was virtually immeasurable. The indirect impacts of depletion of large, strong predators on biodiversity at the community level can thus be enormous and go far beyond a simple reduction in ?-diversity.
Trophic cascades can also happen in pelagic environments. In the Black Sea, the depletion of pelagic predatory fishes, including bonito (Sarda sarda), mackerel (Scomber scombrus), bluefish (Pomatomus saltator), and dolphinfish (Coryphaena hippurus), caused increases in planktivorous fishes. This resulted in a decline of zooplankton and consequently an increase in phytoplankton biomass. It also led to a jellyfish population explosion during the 1970s and 1980s, thereby causing a further decline in zooplankton abundance.
The removal of strong interactors, however, does not always have predictable effects. When predation pressure is released, many other factors can come into play that can prevent an increase in prey abundance. For instance, sea urchin abundance in Mediterranean rocky communities shows a great deal of variance when predatory fish density is low because of the importance of other factors (such as recruitment, larval dispersal, and shelter) once top-down control is lost. The removal of sea otters generally causes an increase in sea urchins and overgrazing of kelp forests in Alaska, but there are healthy kelp forests in California in locales where sea otters are absent and there is no sea urchin harvesting.
Does the removal of weak interactors have a significant effect on biodiversity (Marine biodiversity) at the community level? Paine defined weak interactors as consumers with minimal effects on the dominant prey. On a per capita basis, the absence of weak interactors should not have significant effects on biodiversity. However, the effect of the loss of weak interactors is unpredictable because weak interactions generally show greater variance in their trophic effects than strong interactions. Small species tend to be weak interactors, but these small species have a tendency to have greater densities, which increases their potential food web impacts. Many weak interactors are also being depleted because of hunting and other human-related disturbances.
Changes in tropic relationships: bottom:up
Although top-down trophic cascades are conspicuous results of human harvesting of large predators, other human impacts work from the bottom up. In particular, climate change can result in declines in abundance of species lower in the food web with consequent community-wide bottom:up effects. For instance, the loss of ice in the Southern Ocean in the past 30 years associated with global warming has reduced the amount of algae living under the surface of the sea ice. These algae are a major food source for the krill, Euphasia superba, the abundance of which has dropped by about 80% since the 1970s. In contrast, salps, which are better adapted to warmer waters, have increased in abundance. These changes in prey resources may have been responsible for declines in bird and marine mammal populations in Antarctica and in Antarctic fur seal populations in South Georgia. Such trophic changes can amplify the impact of natural variability in oceanographic conditions.
 The MODIS instrument on Aqua picked out a large phytoplankton bloom off the northern coast of Norway on July 19, 2003. Most of the blue-green color in the (near true color) image was found to be due to Coccolithophore plankton whose carapace (shell) is composed of chalky CaCO3. (Source: NASA)
The MODIS instrument on Aqua picked out a large phytoplankton bloom off the northern coast of Norway on July 19, 2003. Most of the blue-green color in the (near true color) image was found to be due to Coccolithophore plankton whose carapace (shell) is composed of chalky CaCO3. (Source: NASA) Excess nitrogen and phosphorus that is washed seasonally into the sea from rivers also has bottom:up effects on entire communities and eventually results in the creation of dead zones. Massive nutrient inputs in the coastal ocean derived from the use of fertilizers in agriculture provoke huge phytoplankton blooms. When these blooms die and phytoplankton remains fall to the ocean floor, they are decomposed by bacteria that use up the oxygen in the deep-ocean layers. This creates hypoxia and stratification, which prevent the deep water from becoming reoxygenated. When oxygen concentration falls below 2 mg liter?1, most macroscopic marine life (including fish and crustaceans) die or are forced to migrate. Infaunal invertebrates display stress behavior below 1 mg liter?1, and below 0.5 mg liter?1, they decrease in species richness and abundance. The more stressed phases are dominated by a species-poor community composed of a few amphipods, polychaetes, and sipunculids, although macrofauna often exhibits aggregations at the edges of hypoxic zones. The benthic communities in the minimum oxygen zone support low-diversity mats of large sulfide-oxidizing bacteria. This is a case of large-scale loss of biodiversity (Marine biodiversity) at the ecosystem level, where diverse and structurally complex benthic and pelagic communities are turned into simpler microbial communities. Presently, there are about 150 dead zones worldwide, and the number could increase owing to massive and still increasing global use of fertilizer in agriculture.
Fishing at low trophic levels can also reduce the abundance of prey populations and have bottom:up effects on biodiversity. Although in most cases fishing exploits marine [[food web]s] from the top down, there are fisheries associated with productive areas of the ocean such as upwelling regions that are based on species low in the food web, such as sardines, anchovies, and whales. The harvesting of sardines and anchovies acts in synergy with and amplifies fluctuations in biodiversity caused by climate variability. The decimation of the great whales in the northeast Pacific by post-World War II industrial whaling may have caused their natural predators, killer whales (Orcinus orca), to begin feeding successively on seals, sea lions, and finally on sea otters. The removal of oysters can also enhance shifts in diversity and community structure in estuaries and coastal lagoons because of the loss of the filter-feeding function provided by the oysters. On a per capita basis, oysters are weak interactors, but in huge numbers, they can filter the entire volume of bays in days, allowing higher species richness and diversity (Species diversity) of both benthic and planktonic communities. The loss of oysters enhances the homogenization of the ecosystem owing to eutrophication. These bottom:up effects on biodiversity at the ecosystem level are more likely to occur in productive ecosystems, such as upwelling areas or ecosystems in early successional stages, because they have shorter food chain length, greater community-wide turnover (i.e., large production:biomass ratios), and top-down control may be less important.
Changes in habitat/foundation species
Foundation species are generally dominant primary producers and/or habitat-forming species (such as kelps, corals, and mangroves), both in terms of abundance and community influence. Their depletion by human activities, be it directly or through trophic cascades, can have tremendous effects on biodiversity at the community level. For example, the dominant canopy-forming algal species (Cystoseira sp.) in the northwest Mediterranean, described above, sheltered many other species of algae (>100 per 400 cm2), invertebrates, and fish, and the loss of these habitat-creating algae results in an order of magnitude loss of species richness and a decline in ecodiversity. Moreover, the recruitment of many species of littoral fish occurs in shallow bottoms with abundant canopy-forming algae; hence the loss of the algae may also inhibit fish recruitment. In Italy, Guidetti et al. found that the removal of algae associated with the date-mussel Lithophaga lithophaga fishery caused significant changes in species richness and the structure of fish assemblages. The loss of coral cover can also bring about significant changes at the community level. In Papua, New Guinea, Jones et al. found that a devastating decline in coral cover caused a decline in fish biodiversity. Over 75% of reef fish species declined in abundance, and 50% declined to less than half their original abundance, even in no-take areas. The species that suffered the strongest declines where those dependent on living coral as nurseries, and several rare coral specialists became locally extinct. Changes in biodiversity (Marine biodiversity) also occur in deep waters when habitat-forming species are depleted by trawling. Deep corals, sponges, bryozoans, sea pens, and bivalves can provide refuge for predators and prey, provide nursery habitat, and modify the flux of food and larvae at the seafloor. Their loss causes serious declines in biodiversity at all levels.
Changes in tropic relationships and habitat: biological invasions
Invasive species, including certain crabs, snails, and algae, can affect all trophic levels via both top-down and bottom:up effects and by modifying the habitat. Invasive species range from primary producers to sessile benthic invertebrates to zooplankton, although those with the strongest impacts in benthic systems tend to be primary producers or architectural species. For example, the formation of monocultures of the tropical green algae C. taxifolia and C. racemosa in the Mediterranean have caused declines in biodiversity (Marine biodiversity) at the community level. Over 54 species of fishes have colonized the Mediterranean Levant via the Suez canal, and they now account for 13% of the total fish species richness in the region. Another extreme example of community-wide changes after a biological invasion is that which occurred in the Black Sea following the introduction of the small planktonic ctenophore Mnemiopsis. The results have been dramatic reductions in fish biomass, explosive increases of gelatinous zooplankton, and an overall reduced evenness in abundances in the community.
Sometimes, however, invasive species increase biodiversity at small scales. This may occur because an invader consumes the dominant native species or because it provides a habitat that facilitates the colonization of other organisms. In Mission Bay, San Diego, California, the exotic mussel Musculista senhousia builds byssal mats on the surface of soft sediments, which facilitate colonization by other organisms and result in higher abundance and species richness of macrofaunal species compared to the unstructured sediment flats outside the mats. Overall, however, invasions make geographically isolated ecosystems more similar and thus reduce global marine biodiversity.
Synergy of Threats and Global Trends: Homogenization of Marine Biodiversity
Gray stated, “Complete loss of habitat is the most serious threat to marine biodiversity.” Although it is true that total habitat loss would have dramatic effects on marine biodiversity including extinctions, as we have shown here, there are other, more immediate drivers of change that have the potential to erode biodiversity at all levels before habitat is completely lost. These include overfishing, global warming, pollution, and biological invasions. These threats typically act in synergy and produce changes in biodiversity that are more pervasive than those caused by single disturbances. There are local and global disturbances, and all interact at different temporal and spatial scales, creating positive feedback loops that enhance biodiversity loss, diminish resilience, inhibit the recovery of biodiversity, and homogenize marine communities. Human activities can homogenize marine biodiversity via three main processes: (a) by accelerating [[food web]s] (increasing the turnover of communities via fishing down food webs and enhancing microbial activity), (b) by causing pollution-mediated mass mortalities of marine organisms (e.g., dead zones), and (c) by facilitating the dominance of invasive species.
Consequences of biodiversity loss: ecosystem function and services
Marine biodiversity provides most ecosystem services we obtain from the sea, including food security, protection against coastal erosion, recycling of pollutants, climate regulation, and recreation. Biodiversity loss impairs ecosystem services (Marine ecosystem services) from local to global scales. For example, more than half of the catch of the trawl fishery in the Mediterranean coast of Israel now consists of Lessepsian fishes (invaders from the Red Sea through the Suez canal), which have replaced the collapsing populations of native species but with associated declines in productivity. In addition to the obvious declines in [[fisheries]’] productivity, there are many other indirect effects of human-related threats on ecosystem function, including flow of nutrients, resistance to perturbations, stability, and resilience.
Genetic diversity can enhance resistance to disturbance. Hughes & Stachowicz experimentally showed that increasing genotypic diversity in the sea grass Zostera marina enhances community resistance to disturbance by grazing geese. In particular, they found that the number of sea grass shoots remaining in experimental plots after grazing by geese increased with increasing genotypic diversity. However, increased genotypic diversity had no effect on resilience, that is, the rate of shoot recovery after the disturbance.
Species depletions can change ecological processes that are vital to the persistence of marine communities. One example is the effect of biodiversity (Marine biodiversity) on invasion success. Elton suggested that communities with greater species richness should be more resistant to invasion. Recent experimental work has shown that species richness can affect resistance to invasion and thus have significant effects on biodiversity at the community level at small spatial scales. Stachowicz and coauthors have shown that decreasing native species richness in experimental subtidal sessile invertebrate communities increases the survival and final abundance of invaders. Because the abundance of individual species had no effect on invasion success, they suggested that large native species richness reduces invasion success because space is most consistently and completely occupied when more species are present. However, the results of these experiments might be unrealistic because extinctions are generally nonrandom, whereas the studies manipulated species richness by using random subsets of species from a common species pool. Furthermore, although species richness appears to inhibit invasions at small spatial scales, ecosystems with high species richness tend to have more exotic species, suggesting important roles for other factors such as abundance of competitors and predators, productivity, and physical conditions.
However, there appears to be a general pattern of enhancement of stability with an increase in species richness. Emmerson et al. showed, using mesocosm experiments with soft bottom intertidal invertebrates, that effects of species richness on ecosystem function, in this case flux of nutrients (specifically ammonia, NH4-N), are less variable with increasing invertebrate species richness. Declines in species richness alone may thus not be the single most important factor in determining invasion success, and loss of functional biodiversity may be more important. In addition, the homogenization of marine biodiversity mentioned earlier generally means more instability at the community level and consequent boom and bust dynamics, which are not compatible with sustainable exploitation of biodiversity.
The order in which species are lost can govern the ecosystem impacts of biodiversity loss. Modeling work suggests that loss of invertebrate species richness in marine soft sediments leads to a decline in the biogenic mixing depth (BMD), an indicator of bioturbation, which in turn is a primary determinant of species biomass and community structure. However, the pattern of extinction determined the rate of change of the BMD, the species richness at which the BMD first declined, and the variance in the change. For example, the models indicated that losing the large species first led to a faster decline in the BMD compared with random extinction.
Loss of habitat diversity or community diversity may also have dramatic consequences. Mangroves and other coastal communities protect the near shore against erosion from storms and hurricanes. Loss of mangroves causes declines in [[fisheries]’] productivity and amplifies the effects of storms and tsunamis.
Most studies investigating the relationship between biodiversity (Marine biodiversity) and ecosystem function and services (Marine ecosystem services) have manipulated species richness within trophic levels. However, the number of trophic levels, which is related to functional diversity, can be key in determining community-wide biodiversity. This was already apparent in Paine’s classic experimental work in the Pacific northeast intertidal, where the presence of predatory starfishes caused an increase in diversity of major benthic sessile organisms. Recently, Duffy and collaborators showed that, in a sea grass community, higher grazer diversity enhanced ecosystem function (secondary production, epiphyte grazing, and sea grass biomass) only with predators present.
The future of marine biodiversity: the unknown and the unknowable
What we know about biodiversity (Marine biodiversity) change in the past is essential to understanding potential scenarios of change in the future. Identifying the knowable unknowns will help us to identify research priorities and understand the limitations of management.
Recovery of individual species may take longer than expected because of Allee effects, changes in trophic structure of the community (e.g., prey turned predators), difficult-to-reverse habitat changes, or a combination of several factors. Recovery of diversity at the community level will take much longer, probably longer than the generation time of the longest-lived species. In many cases, the reestablishment of native species, in particular trophic specialists, is contingent upon a facilitation process and the provision of minimum biogenic habitat requirements. Even species with high reproductive potential (e.g., the tropical sea urchin Diadema) have been notoriously slow to recover from catastrophic declines, which can have delayed impacts on other ecosystem components with intrinsically slower recovery potential (e.g., corals). In general, recovery of biodiversity is unlikely to happen at global scales as long as the multiple anthropogenic drivers of change are chronic. Because our activities will likely increase in magnitude and extent in the future, we also should expect increasingly frequent collapses and ecosystem shifts.
The globalization of human activities will undoubtedly result in more extinctions, which by definition are irreversible. Some future extinctions may already be inevitable owing to changes that have already occurred (the so-called “extinction debt”), although this provides only very general guidance as to what we might expect given the difficulty of estimating crucial parameters.
The total number of species in marine ecosystems will probably remain unknowable. New molecular techniques developed during the human genome project could allow us to sequence metagenomes and obtain measures of diversity without having to identify and describe every single species. This bar code approach could provide a measure of species richness, including estimates of species loss as a function of gradients in human disturbance. However, there are limits in its ability to provide measures of ecological function. Although function can be assessed for microbes through an assessment of gene expression (metabolic function), ecological function in eukaryotes includes trophic and habitat-forming aspects that may not be predicted by a few genes. Without an understanding of function, it is difficult to know the ecosystem effects of species loss. Natural history and ecological studies to identify what types of species are strong interactors from a community perspective and to identify functional community subsets are hence a priority. In addition, an effort to map marine ecosystems and ecoregions—similar to those conducted for terrestrial ecosystems—will be essential because conserving habitats to preserve species might be an immediate and practical management strategy, regardless of the number of species present.
The relationships between biodiversity (Marine biodiversity), productivity, and stability are often bidirectional, and changes in biodiversity can be both a cause and a consequence of changes in productivity and stability. There is strong evidence of erosion of ecosystem services (Marine ecosystem services) associated to biodiversity declines, although there are still many unknowns, especially at the higher organizational levels and with regard to nonlinearities and feedback loops.
Human population is projected to grow to about 7.5 billion by 2020, with an associated coastal urbanization and migration to the coasts and subsequent increased demand for marine ecosystem services. What will be the global footprint of the new topology of human society? What will be the impact of wealth on biodiversity? The synergies between human drivers, the timing and location of thresholds, the trajectory and timescale of biological adaptation to climate change, and the resilience of marine biodiversity to human perturbations are all unknowns and probably unknowable in detail.
We know with certainty that biodiversity (Marine biodiversity) at all levels will continue to decline locally and to be homogenized globally if human pressure keeps increasing. Ecological theory suggests that the more intact a food web the more likely its recovery after a pulse disturbance. This is based on the fact that biodiversity accumulates slowly over time in a particular location, where production today is used for building structure and adding biodiversity tomorrow. The more production and the more functional components of the food web available, the more likely that a successional trajectory will be reestablished (and hence biodiversity increased) after a disturbance. However, we still do not have an empirical test of this theory for marine communities. The most successful examples of recovery are no-take marine reserves, which generally result in an increase in species richness and biomass of target species, but reserves tend to be small, and the recovery of community-wide biodiversity within their limits is not general.
The direction and the magnitude of change are virtually unpredictable at present because humans are changing the rules of the successional game on a continuous basis. Species go extinct, exotic species are introduced, the physicochemical environment changes continuously, the physical structure of the habitat is altered, and we exert chronic extractive pressure on most trophic levels. All of this occurs at a timescale that is far shorter than the generation time of the largest organisms, which are typically strong interactors and often determine the diversity of entire communities. Merging biodiversity research with food web research may prove particularly productive in developing a science of biodiversity and resilience with practical implications.
Finally, we do not have a good understanding of the relationship between marine biodiversity and ecosystem services (Marine ecosystem services), although there is evidence indicating that more biodiversity can mean either more or less variable services in different circumstances. Increased research in this field is a priority, and it could shed more light into the consequences of biodiversity loss for biodiversity itself and for humans.
Further Reading
- Aldebert, Y., 1997. Demersal resources of the Gulf of Lions (NW Mediterranean): impact of exploitation on fish diversity. Vie Milieu, 47:275–84.
- Atkinson, A., Siegel, V., Pakhomov, E., Rothery, P., 2004. Long-term decline in krill stock and increase in salps within the Southern Ocean. Nature, 432:100–03.
- Babcock, R.C., Kelly, S., Shears, N.T., Walker, J.W., Willis, T.J., 1999. Changes in community structure in temperate marine reserves. Mar. Ecol.-Prog. Ser., 189:125–34.
- Ballesteros, E., 1991. Structure and dynamics of north-western Mediterranean phytobenthic communities: a conceptual model. In Homage to Ramon Margalef, or Why There Is Such Pleasure in Studying Nature, ed. J.D. Ros, N. Prat, pp. 223–42. Barcelona: Publ. Univ. Barcelona. ISBN: 8447500195
- Ballesteros, E., 1992. Els Vegetals i la Zonaci´o Litoral: Esp`ecies, Comunitats I Factors que Influeixen en la Seva Distribuci ´o. Arxius de la Secci´o de Ci`encies, CI. Barcelona: Inst. Estudis Catalans.
- Bambach, R.K., Knoll, A.H,, Wang, S.C., 2004. Origination, extinction, and mass depletions of marine diversity. Paleobiology, 30:522–42.
- Barbier, E.B., 2003. Habitat-fishery linkages and mangrove loss in Thailand. Contemp. Econ. Policy, 21:59–77.
- Barnes, D.K.A., 2002. Biodiversity—invasions by marine life on plastic debris. Nature, 416:808–9.
- Barry, J.P., Baxter, C.H., Sagarin, R.D., Gilman, S.E., 1995. Climate-related, long-term faunal changes in a California rocky intertidal community. Science, 267:672–75.
- Benton, M.J., Twitchett, R.J., 2003. How to kill (almost) all life: the end-Permian extinction event. Trends Ecol. Evol., 18:358–65.
- Berlow, E.L., 1999. Strong effects of weak interactions in ecological communities. Nature, 398:330–34.
- Blokpoel, H., Spaans, L., 1991. Superabundance in gulls: causes, problems and solutions. Presented at Acta XX Congr. Int. Ornithol., Wellington, N. Z.
- Bouchet, P., Lozouet, P., Maestrati, P., Heros, V., 2002. Assessing the magnitude of species richness in tropical marine environments: exceptionally high numbers of molluscs at a New Caledonia site. Biol. J. Linn. Soc., 75:421–36.
- Boudouresque, C.F., Meinesz, A., Ribera, M.A., Ballesteros, E., 1995. Spread of the green alga Caulerpa taxifolia (Caulerpales, Chlorophyta) in the Mediterranean: possible consequences of a major ecological event. Sci. Mar., 59:21–29.
- Boudouresque, C.F., Verlaque, M., 2002. Biological pollution in the Mediterranean Sea: invasive versus introduced macrophytes. Mar. Pollut. Bull., 44:32–38.
- Breitbart, M., Salamon, P., Andresen, B., Mahaffy, J.M., Segall, A.M., et al., 2002. Genomic analysis of uncultured marine viral communities. Proc. Natl. Acad. Sci. USA, 99:14250–55.
- Briggs, J.C., 1994. Species diversity: land and sea compared. Syst. Biol., 43:130–35.
- Bruno, J.F., Petes, L.E., Harvell, C.D., Hettinger, A., 2003. Nutrient enrichment can increase the severity of coral diseases. Ecol. Lett., 6:1056–61.
- Carlton, J.T., Vermeij, G.J., Lindberg, D.R., Carlton, D.A., Dudley, E.C., 1991. The first historical extinction of a marine invertebrate in an ocean-basin: the demise of the eelgrass limpet Lottia alveus. Biol. Bull., 180:72–80.
- Carlton, J.T., Geller, J.B., 1993. Ecological roulette—the global transport of nonindigenous marine organisms. Science, 261:78–82.
- Carlton, J.T., Geller, J.B., Reaka-Kudla, M.L., Norse, E.A., 1999. Historical extinctions in the sea. Annu. Rev. Ecol. Syst., 30:515–38.
- Census of Marine Life
- Chavez, F.P., Ryan, J., Lluch-Cota, S.E., Niquen, M., 2003. From anchovies to sardines and back: multidecadal change in the Pacific Ocean. Science, 299:217–21.
- Christensen, V., 1995. Ecosystem maturity—towards quantification. Ecol. Model., 77:3–32.
- Cohen, A.N., Carlton, J.T., 1998. Accelerating invasion rate in a highly invaded estuary. Science, 279:555–58.
- Coleman, F.C., Williams, S.L., 2002. Overexploiting marine ecosystem engineers: potential consequences for biodiversity. Trends Ecol. Evol., 17:40–44.
- Connell, J.H., 1978. Diversity in tropical rain forests and coral reefs. Science, 199:1302–10.
- Connell, J.H., 1997. Disturbance and recovery of coral assemblages. Coral Reefs, 16: S101–13.
- Connell, J.H., Slatyer, R.O., 1977. Mechanisms of succession in natural communities and their role in community stability and organization. Am. Nat., 111:1119–44.
- Conover, D.O., Munch, S.B., 2002. Sustaining fisheries yields over evolutionary time scales. Science, 297:94–96.
- Crooks, J.A., 1998. Habitat alteration and community-level effects of an exotic mussel, Musculista senhousia. Mar. Ecol.-Prog. Ser., 162:137–52.
- Crouch, E.M., Heilmann-Clausen, C., Brinkhuis, H., Morgans, H.E.G., Rogers, K.M., et al. 2001. Global dinoflagellate event associated with the late Paleocene thermal maximum. Geology, 29:315–18.
- D’Agrosa, C., Lennert-Cody, C.E., Vidal, O., 2000. Vaquita bycatch in Mexico’s artisanal gillnet fisheries: driving a small population to extinction. Conserv. Biol., 14:1110–19.
- Danielsen, F., Sorensen, M.K., Olwig, M.F., Selvam, V., Parish, F., et al., 2005. The Asian tsunami: a protective role for coastal vegetation. Science, 310:643–43.
- Daskalov, G.M., 2002. Overfishing drives a trophic cascade in the Black Sea. Mar. Ecol.-Prog. Ser., 225:53–63.
- Dayton, P.K., 1972. Toward an understanding of community resilience and the potential effects of enrichments to the benthos at McMurdo Sound, Antarctica. In Colloquium on Conservation Problems in Antarctica, ed. B.C. Parker, pp. 81–95. Lawrence, KS: Allen.
- Dayton, P.K., 2003. The importance of the natural sciences to conservation. Am. Nat., 162:1–13.
- Dayton, P.K., Currie, V., Gerrodette, T., Keller, B.D., Rosenthal, R., VenTresca, D. ,1984. Patch dynamics and stability of some California kelp communities. Ecol. Monogr., 54:253–89.
- Dayton, P.K., Tegner, M.J., Parnell, P.E., Edwards, P.B., 1992. Temporal and spatial patterns of disturbance and recovery in a kelp forest community. Ecol. Monogr., 62:421–45.
- Dayton, P.K., Tegner, M.J., Edwards, P.B., Riser, K.L., 1998. Sliding baselines, ghosts, and reduced expectations in kelp forest communities. Ecol. Appl., 8:309–22.
- Dayton, P.K., Thrush, S.F., Coleman, F.C., 2002. Ecological Effects of Fishing in Marine Ecosystems of the United States. Arlington, VA: Pew Oceans Comm.
- Devine, J.A., Baker, K.D., Haedrich, R.L., 2006. Deep-sea fishes qualify as endangered. Nature, 439:29.
- Diaz, R.J., Rosenberg, R., 1995. Marine benthic hypoxia: a review of its ecological effects and the behavioural responses of benthic macrofauna. Oceanogr. Mar. Biol. Annu. Rev., 33:245–303.
- Duffy, J.E., 2002. Biodiversity and ecosystem function: the consumer connection. Oikos, 99:201–19.
- Duffy, J.E., 2003. Biodiversity loss, trophic skew and ecosystem functioning. Ecol. Lett., 6:680–87.
- Duffy, J.E., Richardson, J.P., France, K.E., 2005. Ecosystem consequences of diversity depend on food chain length in estuarine vegetation. Ecol. Lett., 8:301–9.
- Dulvy, N.K., Sadovy, Y., Reynolds, J.D., 2003. Extinction vulnerability in marine populations. Fish Fish., 4:25–64.
- Edmunds, P.J., Carpenter, R.C., 2001. Recovery of Diadema antillarum reduces macroalgal cover and increases abundance of juvenile corals on a Caribbean reef. Proc. Natl. Acad. Sci. USA, 98:5067–71.
- Elton, C.S., 1958. The Ecology of Invasions by Animals and Plants. London: Methuen.
- Emmerson, M.C., Solan, M., Emes, C., Paterson, D.M., Raffaelli, D., 2001. Consistent patterns and the idiosyncratic effects of biodiversity in marine ecosystems. Nature, 411:73–77.
- Erwin, T.L., 1982. Tropical forests: their richness in Coleoptera and other arthropod species. Coleopterists Bull., 36:74–75.
- Estes, J.A., Duggins, D.O., 1995. Sea otters and kelp forests in Alaska: generality and variation in a community ecological paradigm. Ecol. Monogr., 65:75–100.
- Forcada, J., Trathan, P.N., Reid, K., Murphy, E.J., 2005. The effects of global climate variability in pup production of Antarctic fur seals. Ecology, 86:2408–17.
- Foster, M.S., 1975. Algal succession in a Macrocystis pyrifera forest. Mar. Biol., 32:313–29.
- Foster, M.S., Schiel, D.R., 1988. Kelp communities and sea otters: keystone species or just another brick in the wall? In The Community Ecology of Sea Otters, ed.G.R. VanBlaricom, J.A. Estes, pp. 99–115. New York: Springer Verlag. ISBN: 0387180907
- Fukami, H., Budd, A.F., Paulay, G., Sole-Cava, A., Chen, C.L.A., et al., 2004. Conventional taxonomy obscures deep divergence between Pacific and Atlantic corals. Nature, 427:832–35.
- Garcia-Rubies, A., Macpherson, E., 1995. Substrate use and temporal pattern of recruitment in juvenile fishes of the Mediterranean littoral. Mar. Biol., 124:35–42.
- Gaston, K., May, R., 1992.Taxonomy of taxonomists. Nature, 356:281–83.
- Golani, D., 1996. The marine ichthyofauna of the eastern levant—history, inventory, and characterization. Israel J. Zool., 42:15–55.
- Golani, D., Ben-Tuvia, A., 1995. Lessepsian migration and the Mediterranean fisheries of Israel. Conditions of theworld’s aquatic habits. Proc. World Fish. Congr. Theme 1, ed. NB Armantrout, pp. 279–89. New Delhi: Oxford & IBH.
- Goreau, T., 1959. The ecology of Jamaican coral reefs. I. Species composition and zonation. Ecology, 40:67–90.
- Grassle, J.F., Maciolek, N.J., 1992. Deep-sea species richness: regional and local diversity estimates from quantitative bottom samples. Am. Nat., 139:313–41.
- Gray, J.S., 1997. Marine biodiversity: patterns, threats and conservation needs. Biodivers. Conserv., 6:153–75.
- Gray, J.S., 2001. Marine diversity: the paradigms in patterns of species richness examined. Sci. Mar., 65:41–56.
- Gray, J.S., Poore, G.C.B., Ugland, K.I., Wilson, R.S., Olsgard, F., Johannessen, O., 1997. Coastal and deep-sea benthic diversities compared. Mar. Ecol.-Prog. Ser., 159:97–103.
- Grigg, R.W., 1983. Community structure, succession and development of coral reefs in Hawaii. Mar. Ecol.-Prog. Ser., 11:1–14.
- Grigg, R.W., Maragos, J.E., 1974. Recolonization of hermatypic corals on submerged lava flows in Hawaii. Ecology, 55:387–95.
- Grosholz, E.D., Ruiz, G.M., Dean, C.A., Shirley, K.A., Maron, J.L., Connors, P.G., 2000. The impacts of a nonindigenous marine predator in a California bay. Ecology, 81:1206–24.
- Guidetti, P., Fraschetti, S., Terlizzi, A., Boero, F., 2004. Effects of desertification caused by Lithophaga lithophaga (Mollusca) fishery on littoral fish assemblages along rocky coasts of southeastern Italy. Conserv. Biol., 18:1417–23.
- Harmelin-Vivien, M.L., Harmelin, J.G., Leboulleux, V., 1995. Microhabitat requirements for settlement of juvenile sparid fishes on Mediterranean rocky shores. Hydrobiologia, 301:309–20.
- Harvell, C.D., Mitchell, C.E., Ward, J.R., Altizer, S., Dobson, A.P., et al., 2002. Ecology—climate warming and disease risks for terrestrial and marine biota. Science, 296:2158–62.
- Helmuth, B., Harley, C.D.G., Halpin, P.M., O’Donnell, M., Hofmann, G.E., Blanchette, C.A., 2002. Climate change and latitudinal patterns of intertidal thermal stress. Science, 298:1015–17.
- Hoegh-Guldberg, O., 1999. Climate change, coral bleaching and the future of the world’s coral reefs. Mar. Freshw. Res., 50:839–66.
- Hsieh, C.H., Glaser, S.M., Lucas, A.J., Sugihara, G., 2005. Distinguishing random environmental fluctuations from ecological catastrophes for the North Pacific Ocean. Nature, 435:336–40.
- Hughes, A.R., Stachowicz, J.J., 2004. Genetic diversity enhances the resistance of a seagrass ecosystem to disturbance. Proc. Natl. Acad. Sci. USA, 101:8998–9002.
- Huston, M.A., 1985. Patterns of species diversity in relation to depth at Discovery Bay, Jamaica. Bull. Mar. Sci., 37:928–35.
- Hutchings, J.A., Reynolds, J.D., 2004. Marine fish population collapses: consequences for recovery and extinction risk. Bio-Science, 54:297–309.
- IUCN Red List of Threatened Species
- Jackson, J.B.C., 1997. Reefs since Columbus. Coral Reefs, 16:S23–32.
- Jackson, J.B.C., Johnson, K.G., 2001. Paleoecology: measuring past biodiversity. Science, 293:2401–4.
- Jackson, J.B.C., Kirby, M.X., Berger, W.H., Bjorndal, K.A., Botsford, L.W., et al., 2001. Historical overfishing and the recent collapse of coastal ecosystems. Science, 293:629–38.
- Jones, G.P., McCormick, M.I., Srinivasan, M., Eagle, J.V., 2004. Coral decline threatens fish biodiversity in marine reserves. Proc. Natl. Acad. Sci. USA, 101:8251–53.
- Karlson, R.H., Cornell, H.V., Hughes, T.P., 2004. Coral communities are regionally enriched along an oceanic biodiversity gradient. Nature, 429:867–70.
- Kennet, J.P., Stott, L.D., 1991. Abrupt deep-sea warming, palaeoceanographic changes and benthic extinctions at the end of the Palaeocene. Nature, 353:225–29.
- Knoll, A.H., 2001. Life on a Young Planet: The First Three Billion Years of Evolution on Earth. Princeton, NJ: Princeton Univ. Press. 304 pp. ISBN: 0691120293
- Knowlton, N., 1993. Sibling species in the sea. Annu. Rev. Ecol. Syst., 24:189–216.
- Knowlton, N., 2000. Molecular genetic analyses of species boundaries in the sea. Hydrobiologia, 420:73–90.
- Knowlton, N., 2001. The future of coral reefs. Proc. Natl. Acad. Sci. USA, 98:5419–25.
- Knowlton, N., 2004. Multiple “stable” states and the conservation of marine ecosystems. Prog. Oceanogr., 60:387–96.
- Kuntz, N.M., Kline, D.I., Sandin, S.A., Rohwer, F., 2005. Pathologies and mortality rates caused by organic carbon and nutrient stressors in three Caribbean coral species. Mar. Ecol.-Prog. Ser., 294:173–80.
- Large Marine Ecosystems of the World
- LeBoeuf, B.J., Kenyon, K.W., Villa-Ramirez, B., 1986. The Caribbean monk seal is extinct. Mar. Mammal Sci., 2:70–72.
- Lessios, H.A., 1988. Mass mortality of Diadema antillarum in the Caribbean: What have we learned? Annu. Rev. Ecol. Syst., 19:371–93.
- Levin, L.A., 2003. Oxygen minimum zone benthos: adaptation and community response to hypoxia. Oceanogr. Mar. Biol. Annu. Rev., 41:1–45.
- Longhurst, A.R., 2001. Ecological Geography of the Sea. London: Academic. 398 pp. ISBN: 0124555217
- M.J., 2001. Biodiversity on land and in the sea. Geol. J., 36:211–30.
- Magurran, A.E., 2004. Measuring Biological Diversity. Oxford, UK: Blackwell. 256 pp. ISBN: 0632056339
- Malakoff, D., 1997. Extinction on the high seas. Science, 277:486–88.
- Margalef, R., 1963. On certain unifying principles in ecology. Am. Nat., 97:357–74.
- Margalef, R., 1997. Our Biosphere. Oldendorf/Luhe, Ger.: Ecol. Inst.
- May, R.M., 1994. Biological diversity: differences between land and sea. Philos. Trans. R. Soc. London Ser., B 343:105–11.
- McClanahan, T.R., Shafir, S.H., 1990. Causes and consequences of sea urchin abundance and diversity in Kenyan coral reef lagoons. Oecologia, 83:362–70.
- Meinesz, A., 1999. Killer Algae: The True Tale of a Biological Invasion. Chicago, IL: Univ. Chicago Press. 360 pp. ISBN: 0226519236
- Myers, N., Knoll, A.H., 2001. The biotic crisis and the future of evolution. Proc. Natl. Acad. Sci. USA, 98:5389–92.
- Myers, R.A., Worm, B., 2003. Rapid worldwide depletion of predatory fish communities. Nature, 423:280–83.
- Myers, R.A., Worm, B., 2005. Extinction, survival or recovery of large predatory fishes. Philos. Trans. R. Soc. London Ser. B, 360:13–20.
- National Research Council, 1995. Understanding Marine Biodiversity: A Research Agenda for the Nation. Washington, DC: Natl. Acad. 114 pp. ISBN: 0309052254
- Newman, M., 2001. A new picture of life’s history on Earth. Proc. Natl. Acad. Sci. USA, 98:5955–56.
- Norris, S., Rosentrater, L., Eid, P.M., 2002. Polar bears at risk. World Wildl. Fund Rep., Oslo, Norway.
- O’Dor, R., Gallardo, V.A., 2005. How to census marine life: ocean realm field projects. Sci. Mar., 69(Suppl. 1):181–99.
- Odum, E., 1969. The strategy of ecosystem development. Science, 164:262–70.
- Olson, D.M., Dinerstein, E., Wikramanayake, E.D., Burgess, N.D., Powell, G.V.N., et al., 2001. Terrestrial ecoregions of theworlds: a new map of life on Earth. BioScience, 51:933–38.
- Ormond, R., Gage, J., Angel, M., eds., 1997. Marine Biodiversity: Patterns and process. Cambridge, UK: Cambridge Univ. Press. ISBN: 0521022657
- Orr, J.C., Fabry, V.J., Aumont, O., Bopp, L., Doney, S.C., et al., 2005. Anthropogenic ocean acidification over the twenty-first century and its impact on calcifying organisms. Nature, 437:681–86.
- Paine, R.T., 1966. Food web complexity and species diversity. Am. Nat., 100:65–75.
- Paine, R.T., 1980. Food webs: linkage, interaction strength and community infrastructure. J. Anim. Ecol., 49:667–85.
- Paine, R.T., 1992. Food-web analysis through field measurement of per capita interaction strength. Nature, 355:73–75.
- Paine, R.T., 2002. Trophic control of production in a rocky intertidal community. Science, 296:736–39.
- Paine, R.T., Levin, S.A., 1981. Intertidal landscapes: disturbance and the dynamics of pattern. Ecol. Monogr., 51:145–78.
- Palumbi, S.R., 2001. Evolution—humans as the world’s greatest evolutionary force. Science, 293:1786–90.
- Palumbi, S.R., 2001. The Evolution Explosion: How Humans Cause Rapid Evolutionary Change. New York: Norton. 277 pp. ISBN: 0393323382
- Palumbi, S.R., 2003. Marine Reserves: A Tool for Ecosystem Management and Conservation. Arlington, VA: Pew Oceans Commission. ISBN: 0756731534
- Pandolfi, J.M., 2002. Coral community dynamics at multiple scales. Coral Reefs, 21:13–23.
- Pandolfi, J.M., Bradbury, R.H., Sala, E., Hughes, T.P., Bjorndal, K.A., et al., 2003. Global trajectories of the long-term decline of coral reef ecosystems. Science, 301:955–58.
- Pauly, D., Christensen, V., Dalsgaard, J., Froese, R., Torres, F., 1998. Fishing down marine food webs. Science, 279:860–63.
- Perez, T., Garrabou, J., Sartoretto, S., Harmelin, J.G., Francour, P., Vacelet, J., 2000. Mass mortality of marine invertebrates: an unprecedented event in the northwestern Mediterranean. C. R. Acad. Sci. Ser. III Sci. Vie, 323:853–65.
- Perez-Espana, H.F., Arreguin-Sanchez, F., 2001. An inverse relationship between stability and maturity in models of aquatic ecosystems. Ecol. Model., 145:189–93.
- Pimm, S.L., Raven, P., 2000. Biodiversity—extinction by numbers. Nature, 403:843–45.
- Pinnegar, J.K., Polunin, N.V.C., Francour, P., Badalamenti, F., Chemello, R., et al., 2000. Trophic cascades in benthic marine ecosystems: lessons for fisheries and protected-area management. Environ. Conserv., 27:179–200.
- Poore, G.C.B., Wilson, G.D.F., 1993. Marine species richness. Nature, 361:597–98.
- Price, A.R.G., 2002. Simultaneous ‘hotspots’ and ‘coldspots’ of marine biodiversity and implications for global conservation. Mar. Ecol.-Prog. Ser., 241:23–27.
- Quero, J.C., 1998. Changes in the Euro- Atlantic fish species composition resulting from fishing and ocean warming. Ital. J. Zool., 65:493–99.
- Rabalais, N.N., Turner, R.E., Wiseman, W.J., 2002. Gulf of Mexico hypoxia, aka “the dead zone.” Annu. Rev. Ecol. Syst., 33:235–63.
- Raffaelli, D., 2004. How extinction patterns affect ecosystems. Science, 306:1141–42.
- Reaka-Kudla, M.L., 1997. The global biodiversity of coral reefs: a comparison with rain forests. In Biodiversity II: Understanding and Protecting Our Biological Resources, ed. M.L. Reaka-Kudla, D.E. Wilson, E.O. Wilson, pp. 83–108. Washington, DC: Joseph Henry. ISBN: 0309055849
- Reid, K., Croxall, J.P., 2001. Environmental response of upper trophic-level predators reveals a system change in an Antarctic marine ecosystem. Proc. R. Soc. London Ser. B, 268:377–84.
- Rex, M.A., Stuart, C.T., Hessler, R.R., Allen, J.A., Sanders, H.L., Wilson, G.D.F., 1993. Globalscale latitudinal patterns of species diversity in the deep-sea benthos. Nature, 365:636–39.
- Roberts, C.M., 2002. Deep impact: the rising toll of fishing in the deep sea. Trends Ecol. Evol., 17:242–45.
- Roberts, C.M., McClean, C.J., Veron, J.E.N., Hawkins, J.P., Allen, G.R., et al., 2002. Marine biodiversity hotspots and conservation priorities for tropical reefs. Science, 295:1280–84.
- Roemmich, D., McGowan, J., 1995. Climatic warming and the decline of zooplankton in the California current. Science, 267:1324–26.
- Rohwer, F., 2003. Global phage diversity. Cell, 113:141.
- Rohwer, F., Seguritan, V., Azam, F., Knowlton, N., 2002. Diversity and distribution of coral-associated bacteria. Mar. Ecol.-Prog. Ser., 243:1–10.
- Roman, J., Palumbi, S.R., 2003. Whales before whaling in the North Atlantic. Science, 301:508–10.
- Rose, G.A., de Young, B., Kulka, D.W., Goddard, S.V., Fletcher, G.L., 2000. Distribution shifts and overfishing the northern cod (Gadus morhua): a view from the ocean. Can. J. Fish. Aquat. Sci., 57:644–63.
- Ruiz, G.M., Rawlings, T.K., Dobbs, F.C., Drake, L.A., Mullady, T., et al., 2000. Global spread of microorganisms by ships: Ballast water discharged from vessels harbours a cocktail of potential pathogens. Nature, 408:49–50.
- Sala, E., Boudouresque, C.F., Harmelin-Vivien, M., 1998. Fishing, trophic cascades, and the structure of algal assemblages: evaluation of an old but untested paradigm. Oikos, 82:425–39.
- Sala, E., Graham, M.H., 2002. Communitywide distribution of predator-prey interaction strength in kelp forests. Proc. Natl. Acad. Sci. USA, 99:3678–83.
- Sala, E., Sugihara, G., 2005. Food web theory provides guidelines for marine conservation. In Aquatic Food Webs: An Ecosystem Approach, ed. A. Belgrano, U. Scharler, J. Dunne, B. Ulanowicz, pp. 170–83. Oxford, UK: Oxford Univ. Press. ISBN: 0198564821
- Sanders, H.C., 1968. Marine benthic diversity: a comparative study. Am. Nat., 102:243–82.
- Scheffer, M., Carpenter, S., Foley, J.A., Folke, C., Walker, B., 2001. Catastrophic shifts in ecosystems. Nature, 413:591–96.
- Sepkoski, J.J., 1993. Ten years in the library: new data confirm paleontological patterns. Paleobiology, 19:43–51.
- Shea, K., Chesson, P., 2002. Community ecology theory as a framework for biological invasions. Trends Ecol. Evol., 17:170–76.
- Shears, N.T., Babcock, R.C., 2002. Marine reserves demonstrate top-down control of community structure on temperate reefs. Oecologia, 132:131–42.
- Sheehan, P.M., 2001. History of marine biodiversity. Geol. J., 36:231–49.
- Shiganova, T.A., Bulgakova, Y.V., 2000. Effects of gelatinous plankton on Black Sea and Sea of Azov fish and their food resources. ICES J. Mar. Sci., 57:641–48.
- Small, A.M., Adey, W.H., Spoon, D., 1998. Are current estimates of coral reef biodiversity too low? The view through the window of a microcosm. Atoll Res. Bull., 458:1–20.
- Snelgrove, P.V.R., 1998. The biodiversity of macrofaunal organisms in marine sediments. Biodivers. Conserv., 7:1123–32.
- Solan, M., Cardinale, B.J., Downing, A.L., Engelhardt, K.A.M., Ruesink, J.L., Srivastava, D.S., 2004. Extinction and ecosystem function in the marine benthos. Science, 306:1177–80.
- Sousa, W.P., 1979. Experimental investigations of disturbance and ecological succession in a rocky intertidal algal community. Ecol. Monogr., 49:227–54.
- Springer, A.M., Estes, J.A., van Vliet, G.B., Williams, T.M., Doak, D.F., et al. 2003. Sequential megafaunal collapse in the North Pacific Ocean: an ongoing legacy of industrial whaling? Proc. Natl. Acad. Sci. USA, 100:12223–28.
- Stachowicz, J.J., Whitlatch, R.B., Osman, R.W., 1999. Species diversity and invasion resistance in a marine ecosystem. Science, 286:1577–79.
- Stachowicz, J.J., Fried, H., Osman, R.W., Whitlatch, R.B., 2002. Biodiversity, invasion resistance, and marine ecosystem function: reconciling pattern and process. Ecology, 83:2575–90.
- Stachowicz, J.J., Terwin, J.R., Whitlatch, R.B., Osman, R.W., 2002. Linking climate change and biological invasions: Ocean warming facilitates nonindigenous species invasions. Proc. Natl. Acad. Sci. USA, 99:15497–500.
- Stehli, F.G., Wells, J.W., 1971. Diversity and age patterns in hermatypic corals. Syst. Zool., 20:115–26.
- Steneck, R.S., 1998. Human influences on coastal ecosystems: Does overfishing create trophic cascades? Trends Ecol. Evol., 13:429–30.
- Steneck, R.S., Carlton, J.T., 2001. Human alterations of marine communities: Students beware! In Marine Community Ecology, ed. M. Bertness, S. Gaines, M.E. Hay, pp. 445–68. Sunderland, MA: Sinauer. ISBN: 0878930574
- Steneck, R.S., Sala, E., 2005. Large marine carnivores: trophic cascades and topdown controls in coastal ecosystems past and present. In Conserving Predation: Relationships Between Large Carnivorous Animals and Biodiversity, ed. J.C. Ray, K.H. Redford, R.S. Steneck, J. Berger, pp. 110–37. Washington, DC: Island.
- Stocks, K., 2004. Seamount invertebrates: composition and vulnerability to fishing. In Seamounts: Biodiversity and Fisheries. Fisheries Center Research Reports 12(5), ed. T. Morato, D. Pauly, pp. 17–24. Vancouver: Fisheries Centre.
- Tegner, M.J., Basch, L.V., Dayton, P.K., 1996. Near extinction of an exploited marine invertebrate. Trends Ecol. Evol., 11:278–80.
- Thibaut, T., Pinedo, S., Torras, X., Ballesteros, E., 2005. Long-term decline of the populations of Fucales (Cystoseira spp. and Sargassum spp.) in the Alberes coast (France, North-western Mediterranean). Mar. Pollut. Bull., 50:1472–89.
- Thrush, S.F., Dayton, P.K., 2002. Disturbance to marine benthic habitats by trawling and dredging: implications for marine biodiversity. Annu. Rev. Ecol. Syst., 33:449–73.
- Tilman, D., May, R.M., Lehman, C.L., Nowak, M.A., 1994. Habitat destruction and the extinction debt. Nature, 371:65–66.
- United Nations Environment Programme, 2005. GEO year book 2004/5. An overview of our changing environment, Nairobi, Kenya.
- United Nations Population Division, Department of Economic and Social Affairs, UN Secretariat, 2005. Population Prospects: The 2004 Revision. New York: UN.
- Venter, J.C., Remington, K., Heidelberg, J.F., Halpern, A.L., Rusch, D., et al., 2004. Environmental genome shotgun sequencing of the Sargasso Sea. Science, 304:66–74.
- Verlaque, M., 1987. Relations entre Paracentrotus lividus (Lamarck) et le phytobenthos de M´editerran´ee occidentale. Proc. Colloque Int. sur Paracentrotus lividus et les oursins comestibles, ed. C.F. Boudouresque, pp. 5–36. Marseille: GIS Posidonie.
- Verlaque, M., Fritayre, P., 1994. Mediterranean algal communities are changing in the face of the invasive alga Caulerpa taxifolia (Vahl) C. Agardh. Oceanol. Acta, 17:659–72.
- Verlaque, M., Boudouresque, C.F., Meinesz, A., Gravez, V., 2000. The Caulerpa racemosa complex (Caulerpales, Ulvophyceae) in the Mediterranean Sea. Bot. Mar., 43:49–68.
- Vermeij, G.J., 1977. The Mesozoic marine revolution: evidence from snails, predators and grazers. Paleobiology, 3:245–58.
- Ward, J.R., Lafferty, K.D., 2004. The elusive baseline of marine disease: Are diseases in ocean ecosystems increasing? PLoS Biol., 2:542–47.
- Watson, R., Pauly, D., 2001. Systematic distortions in world fisheries catch trends. Nature, 414:534–36.
- Williams, T.M., Estes, J.A., Doak, D.F., Springer, A.M., 2004. Killer appetites: assessing the role of predators in ecological communities. Ecology, 85:3373–84.
- Wommack, K.E., Colwell, R.R., 2000. Virioplankton: viruses in aquatic ecosystems. Microbiol. Mol. Biol. Rev., 64:69–114.
- Worm, B., Duffy, J.E., 2003. Biodiversity, productivity and stability in real food webs. Trends Ecol. Evol., 18:628–32.
- Worm, B., Sandow, M., Oschlies, A., Lotze, H.K., Myers, R.A., 2005. Global patterns of predator diversity in the open oceans. Science, 309:1365–69.
- Yarincik, K., O’Dor, R., 2005. The census of marine life: goals, scope and strategy. Sci. Mar., 69(Suppl. 1):201–8.
