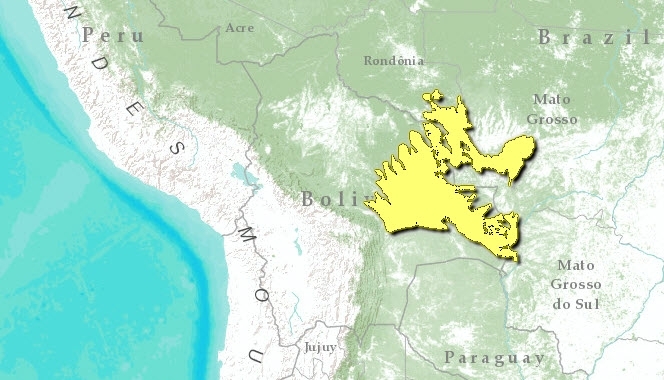
Chiquitano dry forests
TheChiquitano dry forests ecoregion is not only a dry forest but a transition zone between the moist Amazonian forests of the north and these drier forest of the southern Chaco regions, in South America. This dry forest ecoregion harbors extraordinary vertebrate species richness, with a total of 803 vertebrate species. This forest area takes its name from the indigenous groups, Chiquitanos, which inhabited these area at the time of European colonisation. It provided the scenario for most of the Jesuit missionary work during the 17th and 18th centuries.
Location and General Description
Roughly in the center of the South American continent, most of the Chiquitano forest lies within the eastern lowlands of Santa Cruz, Bolivia, with smaller patches extending into western Mato Grosso, Brazil. Situated at the southern limit of Amazonian forests, this forest marks a transition to drier thorny scrub forests further south in the Chaco. Characterized by a strong dry season during the austral winter, the region experiences an annual water deficit of 500 millimeters (mm), notwithstanding a mean annual rainfall of 936 mm. The ancient Brazilian shield underlying the forest presents a gently undulating plain framed by scenic quartzitic ridges and mountains of Precambrian origin aligned in a generally NW to SE direction. Tertiary soils in the peneplain are derived from gneiss and granitic rocks, while Quaternary sediments overlaying the bottom of broad U-shaped valleys such as Tucavaca Valley, which supports the taller forest. Extensive calcareous rocks are found in the Sunsas Ridge, as well as ultramafic soils around Rincon del Tigre, adding to the unique diversity of this area. Rich in hot water springs, some of these contain locally endemic fish such as Bujurquina oenolaemus. Hydrologically, the ecoregion straddles the watershed divide between two major basins in South America, Amazonas and La Plata.
The floristic associations in this forest are very strongly dependent on drainage patterns. The most abundant association "Soto/Curupaú" establishes a 20 meters canopy on well drained soils where the dominant species is Soto or Chamacoco (Schinopsis brasiliensis). Chamacoco is joined by Curupaú (Anadenanthera macrocarpa), Momoqui (Caesalpinia pluviosa), Morado (Machaerium scleroxylon), Roble (Amburana cearensis), Cedro (Cedrela fissilis), and others where they provide approximately 80 percent canopy closure. A five-level strata develops, where emergents reach 30 meters high, with an arboreal, shrubby and herbaceous understory complete the structure.
A second association, "Cuchi/Curupaú" also grows on well-drained but poorer soils. Dependent on establishing on high (steep mountain slopes with rocky soils) or low (sandy piedmont) terrain, the dominant species is Curupaú (Anadenthera macrocarpa) or Cuchi (Astronium urundeuva), respectively. It forms a ten to fifteen meter canopy with 65% coverage where emergents reach 25 meters in height.
A third forest association develops on hygrophilous soils adjacent to creeks that experience shallow flooding during the rainy season. The "Cuta/Ajo-ajo" association has Cuta (Phyllostylon rhamnoides) as the dominant tree species, but Ajo-ajo (Gallesia integrifolia) is a better plant association indicator as it is highly restricted to these flood-prone sites.
A fourth common grouping is the "Tajibo/Tusequi" association formed on small isolated forest islands 0.5-1 meters higher than the surrounding herbaceous savannas. Purple Tabebuia (Tabebuia impetiginosa) is dominant here, while Tusequi (Machaerium isadelphum) is very characteristic as it indicates soils alkalinized through inverse leaching.
Biodiversity Features
Still relatively unstudied, this ecoregion suggests several good candidates for endemic taxa. The ultramafic soils around Rincon del Tigre are conducive to endemism and interesting adaptations in sessile taxa, but have not yet received attention from botanists. The Sunsas Ridge is known to harbor numerous limestone caves rich in bat colonies that have never been studied, for bats or invertebrates. Finally, the difficult access to the isolated grasslands on flat tabletop mountains in the ecoregion has impeded scientific work on them. It was in this forest that Gentry measured plant species richness in the Tucavaca Valley and found the second highest alpha diversity in the world for dry forests.
Surprisingly, the Chiquitano forest is floristically more similar to the geographically distant Caatinga (Caatinga Enclaves moist forests), Misiones and Tucumano forests than its neighbors in the Chaco and Cerrado ecoregions. It appears that the Chiquitano forest functioned as a central node to these currently fragmented dry forest holocene refugia, making it a very promising area for adaptive radiation research.
As headwaters for the Pantanal, the forest is strongly linked to both the flooding and fire regimes typical in grasslands. Many species withstand temporary flooding such as "Ajo-ajo", "Alcornoque" (Tabebuia aurea) and develop fire-resistant bark "Soto", "Verdolago" (Calycophyllum multiflorum). The flooding pulse causes large annual displacements from open grasslands into higher forested land for Marsh Deer (Blastocerus dichotomus) and White-lipped Peccary (Tayassu pecari) (pers. obs.). One extensive block of intact habitat (40,000 square kilometers) in the southeast reaches of the ecoregion remains as the sole guardian of Chiquitano biodiversity. Jaguars, smaller cats and Maned Wolves (Chrysocyon brachyurus) regulate prey populations within an intact biota which boasts healthy populations of Cracids, Tapirs, and Giant Armadillo (Priodontes maximus).
This unique forest shelters a critically endangered reptile , the Broad-snouted Caiman (Caiman latirostris); one endangered bird taxon,Black-and-tawny Seedeater (Sporophila nigrorufa) and at least three special status mammals (Priodontes maximus, Chrysocyon brachyurus and Pteronura brasiliensis), as well as three bird taxa, one reptile taxon and 12 mammalian species in a vulnerable status. Chiquitano forest contained the highest mammalian species richness of several biomes in the ecoregion, with 42 species accounted for in less than two weeks of surveys. In addition, eleven or 26% of those species were threatened or endangered, suggesting that this area is extremely important for harboring rare mammals.
Amphibians in the ecoregion include the Brown-bordered Snouted Treefrog (Scinax fuscomarginatus); Brown-spotted Dwarf Frog (Pleurodema fuscomaculatum); Caceres Robber Frog (Oreobates heterodactylus), known solely from the vicinity of the type locality, Cáceres, in Mato Grosso State, Brazil; Cei's White-lipped Frog (Leptodactylus chaquensis), known for ova deposition in large foam nests on puddles; Chaco Treefrog (Hypsiboas raniceps), typically found on vegetation near ponds or rivers; Chupada Rocket Frog (Allobates brunneus), found in lacustrine margins and stagnant pools; and Labyrinth Frog (Leptodactylus labyrinthicus), tadpoles of which are found in lentic waters.
Current Status
 Santiago Ridge, Santa Cruz, Bolivia. (Source: Photograph by Steffen Reichle/WWF-Bolivia)
Santiago Ridge, Santa Cruz, Bolivia. (Source: Photograph by Steffen Reichle/WWF-Bolivia) This is the largest patch of healthy dry forest ecosystem alive today, and one of the most biologically diverse dry forests in the world. Two large blocks of forest in outstanding conservation condition, comprising 20% of the original ecoregion, remain. Both blocks lie east of San Jose de Chiquitos, split into a north and a south block by a road with ranches along it. Two protected areas Otuquis and San Matias, include important parts of this remnant forest, but linkages between them and effective management are urgent. The Tucavaca Valley is the middle area which needs to be put under protection to provide long-term ecological viability.
Types and Severity of Threats
Dry forests are the most endangered tropical forest in the world and the Chiquitano forest is no exception. Habitat conversion due to agricultural expansion and unplanned colonization is the major threat. Habitat degradation comes next in the form of the Paraguay-Parana Hidrovia Dam project and uncontrolled logging. The third major threat is habitat fragmentation with improved and new access ways promoted by multinational energy companies (pipelines, powerlines and electricity generation) and the transportation sector (roads, ports). Considered globally outstanding for its biological distinctiveness and critically threatened the ecoregion still faces an uncertain future.
Justification of Ecoregion Delineation
The dry forests of the Chiquitano region were deliniated according to national vegetation cover maps. In Brazil, linework follows Instituto Brasileiro de Geografia e Estatística (IBGE) classifications of "semideciduous seasonal submontane forest" and the surrounding "secondary vegetation and agricultural activities" within this broader classification. In Bolivia our linework follows Ribera et al., who classify this region as "region of precambian semideciduous forest (Brazilian shield)".
Further Reading
- Brooks, D.M., J.M. Rojas, H. Aranibar, R.J. Vargas and T. Tarifa. 2001. A preliminary assessment of the mammalian fauna of the Eastern Bolivian Panhandle. Mammalia 65(3).
- Bryant, D., D. Nielsen, & L. Tangley. 1997. The Last Frontier Forests: Ecosystems and Economies on the Edge. WRI Publications, Baltimore, MD. ISBN: 1569731985
- Dinerstein, E., D.M. Olson, D.J. Graham, A.L. Webster, S.A. Primm, M.P. Bookbinder, and G. Ledec. 1995. A conservation assessment of the terrestrial ecoregions of Latin America and the Caribbean. World Wildlife Fund and World Bank. Washington, DC. ISBN: 0821332953
- ENTRIX. 1999. Supplemental Environmental Assessment, Cuiabá Pipeline – Bolivian Portion, Phase I Direct Impacts. ENTRIX, INC. Houston, Texas.
- Ergueta, P., and C. Morales, editors. 1996. Libro Rojo de los Vertebrados de Bolivia. CDC, La Paz, Bolivia.
- Fundação Instituto Brasilero de Geografia Estatástica-IBGE. 1993. Mapa de vegetação do Brasil. Map 1:5,000,000. Rio de Janeiro, Brazil.
- FAN-MBG-MHNNKM-WCS-WWF, 1999. The San Miguel-Cuiabá Pipeline Project. An Independent Supplemental Environmental Assessment. Santa Cruz, Bolivia.
- Gentry, A. 1995. Diversity and floristic composition of neotropical dry forests. Pp. 146-194 in S.H. Bullock, H.A. Mooney, and E. Medina, editors, Seasonally dry tropical forests. Cambridge University Press, Cambridge. ISBN: 0521435145
- Groombridge, B., editor. 1993, 1994. IUCN Red List of Threatened Animals. IUCN, Gland, Switzerland.
- IUCN, 1996. 1996 IUCN Red List of Threatened Animals. IUCN, Gland, Switzerland. 368 pp. ISBN: 2831703352
- Janzen, D. 1988. Tropical dry forests, The most endangered major tropical ecosystem. In E.O. Wilson, Biodiversity. National Academies Press. ISBN: 0309037395
- Killeen, T., E. Garcia, and S. Beck, editors. 1993. Guia de Arboles de Bolivia. Quipus S.R.L., La Paz, Bolivia.
- Montes de Oca, I. 1997. Geografia y Recursos Naturales de Bolivia. La Paz, Bolivia.
- Museo de Historia Natural Noel Kempff Mercado. 1997. Propuesta Técnica para la creación de las áreas protegidas Pantanal de Otuquis y San Matías. MHNNKM, Santa Cruz, Bolivia.
- Parker, T.A., A.H. Gentry, R.B. Foster, L.H. Emmons, and J.V. Remsen, Jr. 1993. The lowland dry forests of Santa Cruz, Bolivia: A global conservation priority. Conservation International, Washington, DC. ISBN: 188117302X
- Prado, D.E., and P.E. Gibbs. 1993. Patterns of species distributions in the dry seasonal forests of South America. Ann. Missouri Botanical Garden. 80: 902-927.
- Ribera, M.O., M. Libermann, S. Beck, and M. Moraes. 1994. Mapa de la vegetacion y areas protegidea de Bolivia. 1:1,500,000. Centro de Investigaciones y Manejo de Recursos Naturales (CIMAR) and Universidad Autónoma Gabriel Rene Moreno (UAGRM), La Paz, Bolivia.
- Tarifa, T., 1996. Mamíferos, Pages 165-264 en P. Ergueta -S. y C. de Morales, editors, Libro Rojo de los Vertebrados de Bolivia CDC-Bolivia.
| Disclaimer: This article contains some information that was originally published by the World Wildlife Fund. Topic editors and authors for the Encyclopedia of Earth have edited its content and added new information. The use of information from the World Wildlife Fund should not be construed as support for or endorsement by that organization for any new information added by EoE personnel, or for any editing of the original content. |