Chemical properties of rivers
| Topics: |
Contents
Introduction (Source: Biodiversity Institute of Ontario (Chemical properties of rivers) )
Water chemistry is responsible for many of the characteristics associated with the "quality" of a river. It is a reflection of complex interdependent relationships involving the river, the atmosphere, the surrounding soil and rocks, groundwater, sediments and living systems.
Rivers have been called the "gutters down which flow the ruins of continents". The world's rivers dump 2.25 x 1010 metric tons of dissolved and particulate matter from erosion of the land into the seas every year, thus playing a major role in global biogeochemical cycling. The river, however, is more than just a transporter of materials. It is also a processor of materials as the biota it contains take up, convert, use and release materials that come to them. It is useful to think of a river as an active biological system that metabolizes the organic matter contained within it. The water that arrives at the mouth of a river is far different, both quantitatively and qualitatively, from what was present in the waters nearer the source.
Distribution of Organic Matter

The metabolic activity of a river depends on two major sources of organic matter: primary production (from living organisms) occurring within the river (autochthonous) and organic matter supplied from the land surrounding the river (allochthonous). It is divided into three general categories: dissolved organic matter (DOM) is carried in solution; coarse particulate organic matter (CPOM) consists of suspended particles greater than 1 millimeter (mm) in size (such as leaves and woody debris); and fine particulate organic matter (FPOM) consists of particles less than 1 mm (such as feces and very small leaf and wood fragments).
The distribution of organic matter is an important component of the river continuum concept . Headwaters tend to have a greater allochthonous production of organic matter. Streamside vegetation usually shades the small streams, preventing much photosynthesis while contributing leaves and woody materials. Thus these headwaters are essentially heterotrophic (that is, they get their "food" from an outside source). The organic matter tends to be quite variable (heterogenous) and coarse (i.e., more CPOM than FPOM). Larger streams down the river continuum have more area exposed to the sun and thus have a greater autochthonous production (i.e., increased photosynthesis). In these streams there is also less input of plant material from the streamside vegetation and thus there is an overall shift to autotrophy (in other words, these streams produce their own "food"). After extensive processing by stream organisms, the organic matter is much finer (i.e., more FPOM and less CPOM) and more uniform (homogenous). By the time the water comes to the large downstream rivers it has "aged" and contains much more total organic matter. This results in more complex aquatic communities. In some cases, however, very large rivers have much greater turbidity resulting in less photosynthesis (because less sunlight penetrates) and become more heterotrophic than the rivers upstream.
Overlain on these general patterns of distribution is extensive daily and seasonal variation in the amount and type of organic matter in rivers. There is also much variation from watershed to watershed.
Nutrient Cycling
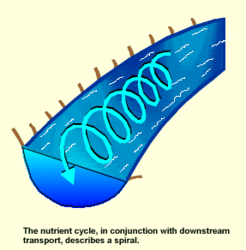
A number of major ions in river water, such as calcium, Magnesium, potassium, sodium and chlorine, are often present well in excess of biological demands within the river and may pass through virtually unaffected. Other elements, however, may be in relatively short supply and undergo considerable utilization as they pass downstream. Most notable among these are the macronutrients nitrogen, phosphorus, and dissolved organic carbon. These compounds are essential for the growth and reproduction of organisms, and rivers are of tremendous importance in their cycling.
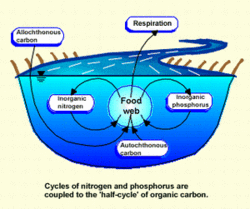
Unlike in standing waters, the cycling of nutrients in [[river]s] does not occur in the same place. A nutrient atom undergoes a series of transformations through aqueous, particulate and consumer compartments as it moves downstream, a process called spiraling. One cycle in a spiral involves the uptake of the nutrient from dissolved organic matter, its passage through the food chain and its return to the water for reutilization. A given nutrient may be used again and again as it passes downstream, the amount of utilization depending on the "tightness" of the spiral. A stream with a tighter spiral, or shorter spiraling length, is more efficient at using and recycling its nutrient resources. A measure called the stream metabolism index (SMI) is sometimes used to compare different stream systems or stream segments.
Variation in Nutrient Load

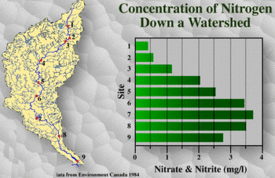
There is tremendous variation in the nutrient loads of [[river]s], both through space and time. For example, nitrogen and phosphorus both accumulate down a watershed as successively larger drainage areas are integrated. These two nutrients also vary significantly with the seasons. Nitrogen and phosphorus concentrations are highest in the late winter and early spring. This is due to factors associated with winter and spring runoffs, such as the release of nutrients stored in snow and ice. These seasonal peaks are most pronounced in the headwaters of the watershed.
The Riparian Zone
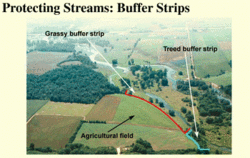
Buffer strips of stream bank vegetation, called riparian zones, are very important in nutrient cycling. The plants serve to "clean" the water before it enters the stream by removing excess nutrients such as nitrogen. This can be particularly important in regions where human activity drastically increases the nutrient load, such as heavily fertilized (Fertilizer) agricultural fields. Riparian vegetation can also be important in reducing erosion of the channel banks.
River Color
The color of water is a function of its chemistry. The transparency and external appearance of a river is largely determined by its sediment load. Generally only springs and mountain streams (i.e., headwaters) are clear, whereas large downstream rivers are usually fairly opaque (cloudy) since light falling on them is reflected by silt particles. Depending on the nature of the suspended particles, the river may take on a characteristic milky-white, greenish, brownish or yellow color. For example, glacial meltwaters are often milky-white due to the presence of large quantities of rock flour. In many cases the color depends on the types of organic materials leached from the surrounding vegetation.
Dissolved Oxygen

The presence of dissolved oxygen is vital to the survival of most aquatic organisms. The dissolved oxygen content (DOC) of a river is subject to physical, chemical and biological controls. The ultimate source of oxygen in water is the atmosphere, although its solubility in river water depends heavily on temperature and pressure. Unpolluted rivers tend to be saturated or even supersaturated with oxygen (100% or more) due to the mixing effect of turbulent flow and the extensive photosynthesis by river plants. The oxygen produced during the day by photosynthesis is consumed by plant and animal respiration, resulting in considerable daily fluctuations in DOC.
There is also seasonal variation corresponding with fluctuations in photosynthesis and temperature (oxygen solubility varies inversely with temperature). Winter ice cover may reduce DOC by reducing photosynthesis and preventing the mixing of air and water. Larger downstream rivers may have reduced DOC because of a greater organic matter content, increased turbidity (causing reduced photosynthesis), and less mixing of the water. Some high mountain streams may also have a lower DOC because of the reduced oxygen solubility associated with the low atmospheric pressures of high altitude. In general, however, headwaters are more saturated with oxygen than lowland rivers.