CFC-11
This article was researched and written by a student at Mount Holyoke College participating in the Encyclopedia of Earth's (EoE) Student Science Communication Project. The project encourages students in undergraduate and graduate programs to write about timely scientific issues under close faculty guidance. This article has also been reviewed by internal EoE editors.
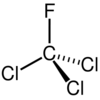

CFC-11, also known as trichlorofluoromethane, Fluorochloroform, Freon 11, CFC 11, R 11, Arcton 9, Freon 11A, Freon 11B, Freon HE or Freon MF was a refrigerant used in operating systems before its ban in 1995. Its chemical formula CCl3F reveals that it is a chlorofluorocarbon, a compound with fluorine (F) and chlorine (Cl) atom attached to the central carbon. TCFC-11 is a very strong radiative forcing gas among the greenhouse gases; possibly, Nitrogen trifluoride is the only stronger greenhouse gas. Both gases have much stronger global warming potential compared to carbon dioxide. Figure 1, and space filled, Figure 2, models are shown below.
Contents
Refrigerants and CFC-11
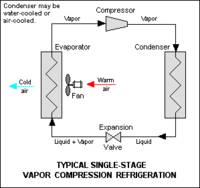
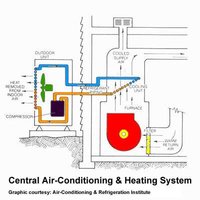
A refrigerant is a compound that can change from a liquid to gaseous state and back without requiring extreme changes in conditions. The phase change from gas to liquid results from pressure build up in the coiled portion an appliance, such as a refrigerator or air conditioner. A small opening (throttle valve) is located in the coil and pressure gets stored there so gaseous CFC-11 changes into liquid, on the other side of the throttle valve the pressure is lower so the boiling point of CFC-11 will become low enough that it will evaporate changing CCl3F(l) to CCl3F(g). The phase change allows for CFC-11 to take heat, as it converts from a liquid to a gas from the surrounding area, especially the inside of the appliance making the refrigerator or air conditioner cold. As CFC-11 moves through the coil pressure is applied by the compressor that cause another phase change from gas to liquid, thus removing more heat from the refrigerator and its surroundings. CFC-11 is an ideal refrigerant to use in air conditioners and refrigerators because of its high boiling point, which puts less stress on an operating system. Figures 3 and 4 depict a refrigerator and an air conditioner operating system respectively.
Ozone and CFC-11
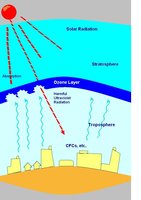
What made CFC-11 so harmful to the environment is its ozone-depleting potential of 1.0, on a scale of 0-1. CFCs are very stable in the troposphere but when CFC-11 hits the stratosphere UV reacts with chlorine atoms in CFC-11 that then react with ozone and initiate cycles that deplete the ozone layer. When this compound is exposed to ultraviolet (UV) light in the stratosphere, chlorine atoms are displaced and CFC-11 splits into CCl2F (gas) + a single chlorine (Cl) in gaseous form. This chlorine atoms reacts with ozone (O3) splitting it to form ClO (gas) + O2 (gas). Since there is a plethora of ozone molecules in the stratospheric layer due to photochemical breakdown of ozone, CFC-11 has the potential to destroy many ozone molecules. Chlorine further breaks down ozone in the atmosphere ClO (g) + 1 oxygen atom (g) into oxygen (O2) and 1 chlorine. This process can occur many times over, one chlorine atom can potentially break apart more that 100,000 ozone molecules! Figure 5 depicts this destructive process.
For more information on the chemistry of ozone depletion, please see the Nobel Lecture Stratospheric Ozone Depletion by Chlorofluorocarbons.
Ban on CFC-11
Banning CFC-11 as a refrigerant was a very long and exhaustive process. The 1987 Montreal Protocol on Substances that Deplete the Ozone Layer was the climax of talks between the United States, Canada and other countries. At this conference an agreement was made to ban and phase out some halogenated hydrocarbons from use. For more information, see Science, diplomacy, and the Montreal Protocol.
CFC-11 was banned in 1995 due to its high ozone depleting potential. Since then organizations such as Green Peace and the Intergovernmental Panel on Climate Change (IPCC) have researched the effects CFC-11 use has had on the ozone layer. Their results indicate that CFC-11 concentrations have decreased since its phaseout but it still lingers in the atmosphere. Table 1 shows the actual concentrations, in parts per trillion, of CFC-11 in the atmosphere from 1992-2006, as reported by researchers at the NOAA Earth System Research Laboratory.
| Table 1. The contribution of different ozone depleting chemicals and groups of chemicals to the ozone-destroying potential of the atmosphere (or Equivalent Chlorine, in parts per trillion or ppt), and the Ozone-Depleting Gas Index relevant for Antarctica (ODGI-A). (Source: Adapted from: NOAA (CFC-11) ) | |||
| Year |
CFC-12 |
CFC-11 |
HCFCs |
| 1992 | 1007 | 813 | 106 |
| 1993 | 1022 | 816 | 111 |
| 1994 | 1035 | 816 | 121 |
| 1995 | 1045 | 814 | 130 |
| 1996 | 1051 | 811 | 140 |
| 1997 | 1057 | 807 | 150 |
| 1998 | 1061 | 801 | 159 |
| 1999 | 1064 | 793 | 169 |
| 2000 | 1068 | 788 | 178 |
| 2001 | 1070 | 782 | 188 |
| 2002 | 1072 | 777 | 197 |
| 2003 | 1072 | 770 | 205 |
| 2004 | 1071 | 764 | 211 |
| 2005 | 1068 | 756 | 218 |
| 2006 | 1065 | 748 | 227 |
Alternatives to CFC-11:
Since CFC-11’s ban in 1995, more environmentally friendly classes of refrigerants are commonly used in its place: hydrochloroflourocarbons (HCFCs) and hydrofluorocarbons (HFCs). According to the U.S. Environmental Protection Agency (EPA), HCFCs “…contain chlorine and thus deplete stratospheric ozone, but to a much lesser extent than CFCs. HCFCs have ozone depletion potentials (ODPs) ranging from 0.01 to 0.1. Production of HCFCs with the highest ODPs will be phased out first US EPA chart below, followed by other HCFCs."
United States Environmental Protection Agency (USEPA), HCFC Phaseout Schedule HCFC Phaseout Schedule, (below):
|
Table 2. Comparison of the Montreal Protocol and United States Phaseout SchedulesMontreal Protocol United States Year to be Implemented % Reduction in Consumption and Production1, Using the Cap as a Baseline Year to be Implemented Implementation of HCFC Phaseout through Clean Air Act Regulations 2004 35.0% 2003 No production and no importing of HCFC-141b 2010 75.0% 65% 2010 No production and no importing of HCFC-142b and HCFC-22, except for use in equipment manufactured before 1/1/2010 (so no production or importing for NEW equipment that uses these refrigerants) 20152 90.0% 2015 No production and no importing of any HCFCs, except for use as refrigerants in equipment manufactured before 1/1/2020 2020 95/5%3 2020 No production and no importing of HCFC-142b and HCFC-22 2030 100.0% 2030 No production and no importing of any HCFCs 1 Adjustments to the HCFC phaseout schedule agreed at the 19th Meeting of the Parties to the Montreal Protocol, September 2007. More details about the September 2007 adjustments to the Montreal Protocol are available here (PDF) (4 pp, 38K, About PDF). 2 The Parties agreed to address the possibilities or need for essential use exemptions, no later than 2015. 3 The Parties agreed to review in 2015 the need for the 0.5 per cent production or import for servicing during the period 2020-2030.Hydrofluorocarbons (HFCs) are another class of refrigerants in use to replace CFC. These compounds do not have chlorine (CFC-11) (Cl) or bromine (Br), which readily react with ozone (O3) to deplete the ozone layer. Although HFCs have an ozone depletion level of 0, according to the US EPA, they have a high Global Warming Potential. |
Many of the HFCs are listed on the US EPA’s Significant New Alternatives Policy (SNAP) Program which publishes a list of All Substitutes for Refrigeration and Air Conditioning.
References:
- Abarca, Janie F., Casiccia, Claudio C. "Skin Cancer and Ultraviolet-B radiation under the Antarctic ozone hole: southern Chile, 1987-2000." Photodermatology, Photoimmunology & Photomedicine, 18(2002): 294-302.
- AUS-e-TUTE. CFC's and ozone depletion.
- Chemical Heritage Foundation. What is a refrigerant?. Faces in Molecular Sciences-Faces in the Environment.
- Dekant, Wolfgang. "Toxicology of Chlorofluorocarbon replacements." Environmental Health Perspectives, 104(1996): 75-83.
- Rhoderick, George C., and William D. Dorko. "Standards Development of Global Warming gas species: Methane, Nitrous Oxide, Trichlorofluoromethane, and Dichlorofluoromethane." Environmental Science & Technology, 2004: 2685-2692.
- Wuebbles, Donald., and Calm, James M. "An Environmental Rationale for Retention of Endangered Chemicals." Science, 27807 Nov 1997 1090-1091.