Ozone
Contents
Introduction
Ozone is a gas made up of three oxygen (Ozone) atoms (O3). It occurs naturally in small (trace) amounts in the upper atmosphere (the stratosphere). Ozone protects life on Earth from the Sun’s ultraviolet (UV) radiation. In the lower atmosphere (the troposphere) near the Earth’s surface, ozone is created by chemical reactions between air pollutants (Air pollution emissions) from vehicle exhaust, motor gasoline vapors, and other emissions. At ground level, high concentrations of ozone are toxic to people and plants.
The word ozone comes from the Greek "ozein" meaning "to smell", and ozone has a characteristic odor that you can detect around high-voltage discharges. A few examples are a photocopy machine, a television or a thunderstorm.
Formation of Ozone in the Troposphere and Stratosphere
Formation of ozone (O3) is very different in the troposphere and stratosphere.
The formation in the troposphere of ozone and related compounds, such as Peroxy-Acetyl Nitrate (PAN) (see Impact of ozone on health and vegetation) is the result of reactions between nitrogen oxides and hydrocarbons. For an overview of global nitrogen oxide and volatile organic compound emissions, see Air pollution emissions.
Volatile organic compounds, such as hydrocarbons and halocarbons, together with nitrogen oxide and solar light (Sunlight), react to ozone and other air pollutants. The light is needed because OH-radicals are, to a large extent, formed out of ozone and the initial step in ozone formation is the reaction of an OH-radical with a hydrocarbon.
Through this process, organic compounds are oxidized and eventually form carbon dioxide and water. Not only ozone and peroxides, but also extra OH radicals, are formed.
The compound chosen here to describe this process is methane, because it is simplest, but in principle all hydrocarbons follow this chain of reaction. Methane is converted first to formaldehyde, then oxidized to carbon monoxide, and finally to carbon dioxide.
In the process, four HO2• radicals are formed. The role of nitrogen oxides is to form back the OH• species. nitric oxide (NO), nitrogen dioxide (NO2) and ozone (O3) are, depending on the amount of light, in equilibrium. The result is a net production of four ozone molecules. Two extra OH• molecules are generated and, importantly, the original NO molecules have reformed. In this process, nitrogen oxides act as a catalyst, not as a reagent, and one nitrogen oxide can form many ozone molecules until it disappears, mainly by conversion to nitric acid.
The oxidation of hydrocarbons to carbon dioxide and water has environmental advantages; this mechanism is responsible for the destruction of toxic organic compounds that would otherwise be a much greater hazard than at present, because their lifetimes in the environment would be much longer.
At higher altitudes, in the stratosphere, high concentrations of ozone are present and this ozone is produced by a different mechanism (see Figure 1).
Oxygen molecules, O2, are split by shortwave UV and the resulting oxygen atoms react with oxygen molecules to form ozone (see Figure 2).
This ozone absorbs most of the shortwave UV radiation present in the total solar radiation. This UV absorption heats the stratosphere. In fact, all UV-C (wavelength < 290 nm) radiation and a large part of UV-B (wavelength 290 to 320 nm) radiation is absorbed.
Going up in the troposphere, the temperature drops as a function of adiabatic expansion (temperature in a gas goes down if the pressure is lowered without heat exchange) and less heat transport from the surface (see Figure 3). The surface is heated by solar radiation and this heat from the surface is redistributed in the troposphere by turbulent transport, when the heated surface creates turbulent eddies. This transport is smaller further away from the surface. A temperature minimum of –50 to –80°C is reached at the tropopause.
The height of the tropopause varies with latitude. Due to much higher solar fluxes, the tropopause is at a height of 17 km in the tropics and about 10 km in the polar regions. The warmer, lighter air sitting atop the relatively colder troposphere prevents easy mixing of tropospheric air with stratospheric air, hence the name tropopause.
Looking closely at Figure 1, a small hump in ozone concentrations is seen near the tropopause, the cold layer that separates the troposphere and stratosphere. This ozone, acting as a greenhouse gas (see below), has drawn a lot of attention, see Impact of ozone on climate change.
Overview of Problems Caused by Ozone
Ozone is at the core of many environmental problems, but these effects are very different depending on where they are encountered in the troposphere and stratosphere (see Figure 4). High levels of ozone and other oxidants such as peroxy-acetyl nitrate (PAN) were clearly becoming a threat to human and ecosystem health (Ozone) in many cities throughout the United States. Cities such as Los Angeles, California, where stagnant meteorological episodes with minimal air exchange are often encountered, were most affected. This ozone problem is becoming more widespread as transportation emissions have intensified.
Ozone is a greenhouse gas; it absorbs infrared radiation and re-emits it as radiation at an energy level equivalent to about 18°C. This means that the impact of ozone, at temperatures encountered at the surface, is minimal, with respect to greenhouse forcing. However tropospheric ozone has played a significant role in global warming, due to depletion of high altitude depletion of ozone. Prior to the Montreal Protocol ozone depletion contributed to global warming by increasing solar radiation of the Earth; subsequent to Montreal, the ozone hole has been healing and contributing thus to a global cooling effect. Ozone is a much more effective greenhouse gas at the tropopause, where temperatures of –60 to –80°C are encountered. For more information, see Impact of ozone on climate change.
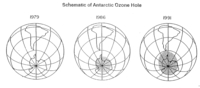
From satellite images and Dobson Spectrometer measurements, it became clear in the period around 1980–1985 that a substantial portion of stratospheric ozone in Antarctica was disappearing during the local springtime (see Figure 5 and Antarctic ozone hole for more information). (A Dobson Spectrometer measures solar light intensity at a wavelength at which ozone absorbs light, and at one where ozone does not absorb light, and from the difference derives the total ozone column between the apparatus and the sun.)
In Figure 5, the expanding and darker color of the spot over Antarctica describes the scale and intensity of ozone loss in the stratosphere. Less stratospheric ozone means that more shortwave UV radiation will reach the Earth's surface. This would not only affect human health (Impact of ozone on health and vegetation) by causing skin cancer, but could potentially affect marine ecosystems (Marine ecosystem services) by damaging algae populations, the basis of the oceanic food chain. Moreover, it was feared that the problem would not be restricted to Antarctica but would become an environmental threat on a global scale.
The striking observation can been made that there is excessive ozone in the lower and higher troposphere, causing damage to human and ecosystem health and amplifying the greenhouse effect respectively, while ozone is disappearing in the stratosphere, where it acts as an essential filter for UV-B and UV-C radiation. Approximately 90% of total ozone is in the stratosphere and the remaining 10% in the troposphere. Hence, the increase in tropospheric ozone cannot compensate for the loss in the stratospheric ozone.
References
- Antarctic climate and environment history in the pre-instrumental period. (2021) Editors: Luca Bargelloni, John Turner et al. Victoire Press. Cambridge, UK. Published by the Scientific Committee on Antarctic ResearchScott Polar Research Institute, Lensfield Road,Cambridge, UK. ISBN 978-0-948277-22-1
- Intergovernmental Panel on Climate Change, United Nations Environment Programme. Technology and Economics Assessment Panel. 2005. IPCC/TEAP special report on safeguarding the ozone layer and the global climate system: issues related to hydrofluorocarbons and perfluorocarbons. Cambridge University Press. 478 pages
- Arjun Makhijani, Kevin Gurney. 1995. Mending the ozone hole: science, technology, and policy. MIT Press. 355 pages
See Also
Citation
Sjaak Slanina (2012). Ozone. eds. Howard Hanson & C. Michael Hogan. Encyclopedia of Earth. National Council for Science and Environment. Washington DC. Retrieved from http://editors.eol.org/eoearth/Wiki/Ozone