Matter
| Topics: |
Matter is the material that makes up objects in the Universe. All matter on the Earth is constructed of elements (see periodic table of elements). Chemists have described approximately 115 different elements. Each of these elements have distinct chemical characteristics. Table 1 lists some of the chemical characteristics for 48 common elements found in the Earth's continental crust.
The smallest particle that exhibits the unique chemical characteristics of an element is known as an atom. Atoms are composed of yet smaller particles known as protons, neutrons, and electrons. A proton is a subatomic particle that has significant mass and contributes a single positive electrical charge to an atom. Neutrons also have significant mass but no electrical charge. Electrons are extremely light subatomic particles having a mass that is 1/1840 of a proton. Each electron also has a negative electrical charge.
Protons and neutrons make up the nucleus of an atom. As a result, most of an atom's mass is concentrated in the nucleus. Because protons are positively charged, the nucleus has a positive charge equal to the number of these subatomic particles. Electrons are found orbiting outside the nucleus at various distances based on their energy level. The area occupied by the electrons has a negative charge equal to the number of these subatomic particles. If an atom has an equal number of electrons and protons its net electrical charge is zero. If there are more electrons than protons the charge of the atom is negative. Likewise, if there are less electrons than protons the charge of the atom is positive. In both cases, the exact charge is determined by subtracting protons from electrons. As a result, 4 protons minus 6 electrons give an atomic charge of -2.
The number of protons found in the nuclei of the different types of elements is unique and is referred to as the atomic number (Table 1). All atoms of a specific element have the same number of protons in their nuclei. Atomic mass number is an atom's total number of neutrons and protons. Many elements have unequal numbers of neutrons and protons in their nucleus. An element's atomic weight refers to the total weight of neutrons, protons, and electrons. For example, the atomic weight of aluminum is 26.98 (Table 1). Atomic number describes the number of protons found in an atom. For example, silver has an atomic number of 47 since it has 47 protons in its atom (Table 1). Some elements can have variants containing different numbers of neutrons but similar numbers of protons. We call these variants isotopes. Carbon has two isotopes. Its most common form is carbon-12 which has 6 protons plus 6 neutrons. About 99% of the carbon on our planet is of this type. The isotope carbon-13 has 6 protons plus 7 neutrons. Carbon-14 is the rarest isotope of carbon containing 8 neutrons. Some isotopes are unstable and their nucleus tends to lose subatomic particles forming an element with a lower atomic mass. This process is known as radioactive decay.
|
Table 1: Characteristics of some of the common chemical elements found in the Earth's continental crust. | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Element | Chemical Symbol |
Atomic Number |
Common Atomic Mass Number |
Atomic Weight |
Percent in Continental Crust |
Required for all Life |
Required for Some Lifeforms |
Element Type |
Moderately Toxic |
Extremely Toxic |
| Al | 13 | 27 | 26.98 | 8.2300 | - | X | Metalloid | - | - | |
| Sb | 51 | 122 | 121.75 | 0.00002 | - | - | Metalloid | - | - | |
| As | 33 | 75 | 74.92 | 0.00018 | - | - | Metalloid | - | X | |
| Barium | Ba | 56 | 137 | 137.34 | 0.0425 | - | - | Metal | - | - |
| Be | 4 | 10 | 9.01 | 0.00028 | - | - | Metal | - | X | |
| Bi | 83 | 209 | 208.98 | 0.000017 | - | - | Metal | - | - | |
| B | 5 | 11 | 10.81 | 0.0010 | - | - | Metalloid | - | - | |
| Br | 35 | 80 | 79.91 | 0.00025 | - | - | Nonmetal | - | - | |
| Cd | 48 | 112 | 112.40 | 0.00002 | - | - | Metal | - | X | |
| Calcium | Ca | 20 | 40 | 40.08 | 4.1000 | X | - | Metal | - | - |
| C | 6 | 12 | 12.01 | 0.0200 | X | - | Nonmetal | - | - | |
| Cl | 17 | 35.5 | 35.45 | 0.0130 | - | X | Nonmetal | X | - | |
| Cr | 24 | 52 | 52.00 | 0.0100 | - | - | Metal | X | - | |
| Co | 27 | 59 | 58.93 | 0.0025 | - | X | Metal | - | - | |
| Cu | 29 | 63.5 | 63.54 | 0.0055 | X | - | Metal | X | - | |
| F | 9 | 19 | 19.00 | 0.0625 | - | X | Nonmetal | X | - | |
| Ga | 31 | 70 | 69.72 | 0.0015 | - | - | Metal | - | - | |
| Ge | 32 | 73 | 72.59 | 0.00015 | - | - | Metalloid | - | - | |
| Au | 79 | 197 | 196.97 | 0.0000004 | - | - | Metal | - | - | |
| H | 1 | 1 | 1.008 | 1.4000 | X | - | Nonmetal | - | - | |
| I | 53 | 127 | 126.90 | 0.00005 | - | X | Nonmetal | - | - | |
| Fe | 26 | 56 | 55.85 | 5.6000 | X | - | Metal | - | - | |
| Pb | 82 | 207 | 207.19 | 0.00125 | - | - | Metal | - | X | |
| Li | 3 | 6 | 6.94 | 0.0020 | - | - | Metal | - | - | |
|
Magnesium |
Mg | 12 | 24 | 24.31 | 2.3000 | X | - | Metal | - | - |
| Mn | 25 | 55 | 54.94 | 0.0950 | X | - | Metal | - | - | |
| Hg | 80 | 201 | 200.59 | 0.000008 | - | - | Metal | - | X | |
| Mo | 42 | 96 | 95.94 | 0.00015 | X | - | Metal | - | - | |
| Ni | 28 | 59 | 58.71 | 0.0075 | - | - | Metal | - | X | |
| N | 7 | 14 | 14.01 | 0.0020 | X | - | Nonmetal | - | - | |
| O | 8 | 16 | 16.00 | 46.4000 | X | - | Nonmetal | - | - | |
| Palladium | Pd | 46 | 106 | 106.40 | 0.000001 | - | - | Metal | X | - |
| Phosphorus | P | 15 | 31 | 30.97 | 0.1050 | X | - | Nonmetal | - | - |
| Pt | 78 | 195 | 195.09 | 0.0000005 | - | - | Metal | - | - | |
| K | 19 | 39 | 39.10 | 2.1000 | X | - | Metal | - | - | |
| Rb | 37 | 85.5 | 85.47 | 0.0090 | - | - | Metal | - | - | |
| Se | 34 | 79 | 78.96 | 0.000005 | - | X | Nonmetal | X | - | |
| Si | 14 | 28 | 28.09 | 28.2000 | - | - | Metalloid | - | - | |
| Ag | 47 | 108 | 107.87 | 0.000007 | - | - | Metal | - | X | |
| Sodium | Na | 11 | 23 | 22.99 | 2.4000 | - | X | Metal | - | - |
| Sulfur | S | 16 | 32 | 32.06 | 0.0260 | X | - | Nonmetal | - | - |
| Th | 90 | 232 | 232.04 | 0.00096 | - | - | - | - | - | |
| Tin | Sn | 50 | 119 | 118.69 | 0.00020 | - | - | Metal | X | - |
| Ti | 22 | 48 | 47.90 | 0.5700 | - | - | Metal | - | - | |
| W | 74 | 184 | 183.85 | 0.00015 | - | - | Metal | - | - | |
| U | 92 | 238 | 238.03 | 0.00027 | - | - | - | - | - | |
| V | 23 | 51 | 50.94 | 0.0135 | - | X | Metal | X | - | |
| Zn | 30 | 65 | 65.37 | 0.0070 | X | - | Metal | - | - | |
Elements can be classified as being either metals, nonmetals, or metalloids (Table 1). Metals are Elements (Matter) that usually conduct heat and electricity and are shiny. Nonmetals do not conduct electricity as well and are normally not shiny. Metalloids have characteristics that are in between metals and nonmetals.
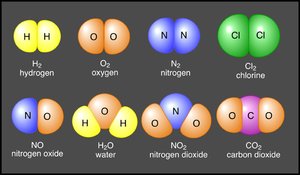
Elements with a net positive or negative charge are called ions. Chemists indicate the number of positive or negative charges on an ion using a superscript after the element's symbol. For example, calcium has two positive charges and is written as Ca2+. Some common negatively charged ions include nitrate (NO3-), sulfate (SO42-), and phosphate (PO43-).
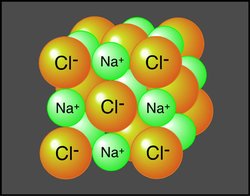
Positive and negative ions are electrically attracted to each other. This mutual attraction allows for the bonding of [[atom]s] to occur forming structures of matter that are larger than just one atom. When similar atoms bond together they construct molecules. Atoms of different elements joined together form compounds (Figure 1). Sodium chloride (or table salt), is an ionic compound consisting of sodium (Na+) and chloride (Cl-). In nature, it forms as a three-dimensional array of oppositely charged ions (Figure 2). Many of the Earth's substances have a molecular structure similar to sodium chloride.
Further Reading: PhysicalGeography.net