
|
Flies and Mosquito Borne Diseases
|
| Introduction | Trypanosomiasis / Nagana | |||
| 3-Day Sickness (Ephemeral Fever) | Review Process | |||
| Bluetongue | Information Source Links | |||
| Rift Valley Fever |
Introduction
3-Day Sickness (Ephemeral Fever)
Common Names: Three Day Sickness, Stiff Sickness
Description: Flies and mosquito borne disease
Introduction
Ephemeral Fever is an insect-transmitted, non-contagious, viral disease of cattle and water buffalo found in Africa, the Middle East, Asia south of the former USSR, and Australia. Infections with no illness can also occur in wild buffalo, waterbuck, hartebeest, wildebeest, deer and possibly goats.
Mode of Spread
The virus is insect-borne and is not contagious. This virus does not persist in recovered cattle and most recovered cattle have life-long immunity. The insects responsible for spread are several species of midge and mosquitoes. Outbreaks may be more common when conditions favour multiplication of the midge and mosquito populations. Wind may spread the responsible midges over large areas.
The prevalence and severity of the disease may vary from year to year. The disease tend to disappear for long periods, to return when the resistance of the population is diminished. Recurrence depends primarily on suitable environmental conditions for the increase and dissemination of the insect vector. During periods of inactivity, the disease is still present but the number of animals affected is very low. Clinical cases may occur without being recognised. Outbreaks may depend on a change in the virulence of the virus or an increase in the insect population.
During epidemics many animals may be affected within days or 2-3 weeks.
Signs of Ephemeral Fever
Signs
- After an incubation period of 2-10 days the virus causes inflammation in tissues such as joints, muscles and lymph nodes.
- There is a fever of up to 40.5°C - 41°C (105°F - 106°F) lasting for 2 days, an increased respiratory rate, difficulty in breathing, muscle stiffness and pain.
- There is a sudden decrease in milk yield. (After recovery milk production does not return to normal levels until the following lactation.)
- Affected animals refuse to eat, drool saliva from the mouth, have difficulty in swallowing and have muscle tremors.
- There is often an excessive production of tears, a nasal discharge and occasionally constipation or diarrhoea.
- The most characteristic signs, however, are the stiffness or lameness. Some animals become recumbent (lie down) and paralyzed from 8 hours to a week. In severe cases animals suffer permanent paralysis, but this is uncommon. Above picture shows heifer with BEF virus unable to bear weight on its hind legs.
- In most cases spontaneous recovery occurs after about 2 days, and is complete.
Bulls, heavy cattle, and high-yielding dairy cattle are the most severly affected, with animals less than 6 months of age showing no clinical signs. Pregnant cows may abort, especially those in late pregnancy. Bulls may suffer temporary infertility.
Diagnosis
This is based on the clinical signs. This should pose no problem during an outbreak but can cause difficulties in isolated cases, when it may be confused with Laminitis, milk fever or a foreign body in the reticulum. After a few days however, the spontaneous improvement should aid diagnosis.
Prevention - Control - Treatment
Prevention and Control
Control of the insect carriers is not possible. Inactivated and live virus vaccine combinations are used in some parts of the world, such as South Africa and Australia and appear to be effective. Such vaccines should, however, only be used in endemic areas.
Treatment
Generally, no treatment is required, but in individual cases anti-inflammatory drugs, such as injectable phenylbutazone or flunixin will help recovery. Animals should be rested and not stressed, as this may cause the disease to come back. No drugs should ever be given by mouth to an animal with Ephemeral Fever as many animals are unable to swallow and to do so will cause pneumonia. Antibiotic treatment to control secondary infection and rehydration with isotonic fluids may be warranted in some cases.
Bluetongue
 |
| Bluetongue |
| © Geoffrey P. Gard. Reproduced from the Animal Health and Production Compendium, 2007 Edition. CAB International, Wallingford, UK, 2007 |
Description:Flies and mosquito borne disease
Introduction
Bluetongue is an infectious, non-contagious, insect-borne disease, primarily of domestic and wild ruminants. Sheep and cattle are most commonly affected.
It is characterized by fever, inflammation of the nose, lips and muzzle (ulcerative stomatitis), muscle oedema and lameness due to inflammation of the hooves.
The disease is common worldwide wherever conditions favour the survival of the responsible vector.
There are 24 different serotypes of Bluetongue viruses worldwide but not all exist in one geographical area. The virus is very stable and resistant and can survive for years at most shade temperatures.
Bluetongue is a potentially very serious disease in sheep. The greatest loss sustained by the owner is an indirect one owing largely to the very marked loss in condition, the long recovery period and the break in wool, which are the inevitable results of an attack of Bluetongue. Many affected sheep remain in such a state of weakness following infection that many weeks or months of good feeding may be required before they regain condition. Awareness of this disease and how to combat it should be part of the armoury of every sheep farmer.
 |
| Female Culicuides Midge |
| © Philip Mellor |
Bluetongue is spread by the Culicoides biting midge (small flies), which is Culicoides imicola in Africa, Southern Europe and the Middle East. Mechanical transmission by other bloodsucking insects is over minor significance.
Infected Culicoides prefer warm, moist conditions and are highly nomadic, moving over vast distances thereby allowing the virus access to a constant supply of susceptible hosts.
Bluetongue is prevalent in seasons when the Culicoides are most active. The virus has a great attraction to blood cells and the Cuilcoides become infected by blood from infected animals, about 10 days after sucking the infected blood.
Cattle act as carriers because of the extended period of presence of virus in the blood in cattle - up to 9 weeks - and the host preference of most Culicoides for cattle, allowing for year round transmission in domestic ruminants.
Active illness is limited almost exclusively to sheep, but cycles silently without symptoms in other infected ruminants, wild and domesticated, including camels.
The importance of the disease in cattle may be that they form a reservoir of infection, which may be passed on to sheep. Sheep that survive develop antibodies and an immune response to the virus type that infects them, but they remain susceptible to a second virus type. However there is some cross-protection between some of the virus types. Cattle, when infected, undergo persistent and later hidden infections, lasting about 50 days.
Evidence of infection with Bluetongue virus has been found in large carnivores in Africa, perhaps as a result of eating virus-infected inner animal parts.
The disease is not contagious. However, semen from infected bulls can serve as a source of infection. Congenital abnormalities and foetal death can occur due to transmission from mother to offspring.
British breeds of sheep and Merinos appear to be more susceptible to Bluetongue than do native African sheep. Young sheep about a year old are most susceptible.
Exposure to a lot of sunshine seems to increase the severity of the disease.
Signs of Bluetongue
- The incubation period is about a week. Onset of illness is manifested by a high fever, which fluctuates for about a week.
- Together with the fever there is inflammation of the lips and nose, excessive salivation with licking of the lips and a clear nasal discharge. Within a few days the discharge has mucous and pus and is bloodstained. It then dries, encrusting the nostrils.
- Meanwhile the lips swell and are very tender and bleed when handled. Sometimes a swollen, blue tongue is evident. The face swells. Deep ulcers, filled with white, dead debris appear at any site where there is irritation.
- Towards the end of the fever, lameness and stiffness develop. The lameness is due to inflammation at the coronary band of the hoof (where the hoof meets the skin of the leg), manifesting as a red or purplish band which travels down the hoof with the growth of horn. The stiffness is due to degeneration of skeletal muscle fibres. Severely affected animals lie down and will only move on their knees if forced.
- Twisting of the neck, may occur due to the direct effect of the virus on muscle tissue. After this, emaciation and weakness are rapid.
- Secondary infections of the respiratory tract are common and diarrhea, often bloodstained, is sometimes seen.
- The mortality rate varies from 0 to 30%, although, in isolated epidemics it may reach 90%. Severely affected animals die within a week of the onset of fever, but others may linger on and only die after a month.
- Recovery in surviving sheep is prolonged and such animals frequently exhibit a moth-eaten appearance caused by breaks in the wool.
- The disease in cattle is frequently inapparent, although a few animals may develop signs similar to that seen in severely infected sheep. These include fever of 40°C to 41°C, stiffness, laminitis (damage to the sensitive tissue of the hooves), excessive salivation, swelling of the lips, loss of appetite, nasal discharge and a bad smelling breath. Ulcerative lesions may appear on the tongue, dental pad and muzzle. A severe inflammation of the coronet of the hoof may occur, sometimes with sloughing of the hoof. A discharge may appear at the nostrils and from the eyes. Wounds may occur on the teats.
- Infected goats show little in the way of clinical symptoms.
- Cattle and sheep infected during pregnancy may abort or deliver malformed calves or lambs.
Diagnosis
The history, clinical signs, and post mortem findings are highly suggestive and should enable a presumptive diagnosis to be made.
Changes in the alimentary tract are most prominent in the mouth, where the mucous membranes are fluidfilled, have accumulated blood and sometimes have a bluish discoloration due to shortage of oxygen. Discharge and flakes which are often covered with gray dead tissue are present on the lips, hard palate, cheeks and tongue. Congestion, inflammation of mucous membranes, and slight bleeding occur throughout the small intestine. The nostrils are partly blocked by encrusted nasal discharge. The upper respiratory tract exhibits an inflammation of mucous membranes and occasionally the lungs have fluid.
Skin and muscles show the most constant and most suggestive lesions. Exposed areas of skin have irregular encrusted eruptions. The subcutaneous and intramuscular connective tissues are infiltrated with a red gelatinous fluid. Tiny areas of bleeding are found scattered throughout the muscles, together with gray areas of dead cells producing a mottled appearance.
In order to confirm the presumptive diagnosis a veterinary surgeon will take samples for analysis. From live animals coagulated and non-coagulated blood will be taken. From dead animals spleen and mesenteric lymph nodes will be taken and sent on wet-ice to a laboratory. Blood samples should not be frozen.
Bluetongue must be differentiated from other diseases which may have some symptoms in common. These include Foot and Mouth Disease, Sheep and Goat Pox and Orf in sheep and Foot and Mouth Disease, Ephemeral Fever, Sweating Sickness in cattle, and Goat Plaque.
Prevention - Control - Treatment
Prevention and Control
- Control is primarily based on vaccination, but other measures will help to reduce the incidence of infection. Control of the Culicoides that spread Bluetongue is difficult, but infection can be avoided by moving stock during the rains to high, well-drained ground where there are no Culicoides or by housing in sheds where lights, which might attract insects, are not permitted at night.
- Smoke fires at night and use the insecticides have a role to play and housing cattle together with sheep on the grounds that Culicoides prefer cattle blood to sheep blood should be considered.
- Yearly vaccination of sheep with a live attenuated vaccine containing six strains of Bluetongue virus, available from KEVEVAPI, Nairobi, is recommended, and will do much to keep losses to a low level. It will not eradicate the disease as the virus persists in inapparent carriers in wild ruminants and other strains of the virus not incorporated in the vaccine may be present in the locality.
- Lambs under three months should not be vaccinated nor should pregnant ewes, under normal circumstances.
Treatment
There are at present no drugs which have a curative effect on Bluetongue. Antibiotics however, have a beneficial effect in combating secondary bacterial infections.
Careful nursing of affected animals is important. Affected animals should be placed in sheds or stables and protected from extreme temperature and from direct sunlight. Small quantities of soft green feeds should be given during the stage when mouth lesions make feeding painful.
Secondary invaders can be controlled by the application of lotions to the wounds. During convalescence it is most important that ruminal activity be maintained by suitable measures. Once this has been achieved, careful and good feeding will reduce the recovery period and materially assist in re-establishing normal health.
Rift Valley Fever
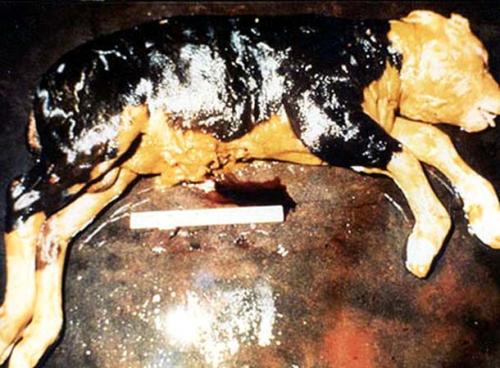 |
| Aborted foetus after Rift Valley Fever |
| © USDA |
Description: Mosquito borne disease
Introduction
Rift Valley Fever (RVF) is an accute or peracute, mosquito-borne viral disease affecting domestic ruminants (cattle, sheep, goats, camels, domestic buffaloes) and man. It occurs mainly in East and Southern Africa and in year 2000 in Saudi Arabia and Yemen. During epidemics the occurrence of numerous abortions, deaths in young animals and influenza like diseases in humans tends to be characteristic.
Formerly, apart from abortion, most cases in adult animals were subacute, but in recent times a haemorrhagic form of the disease has emerged with rapid death in mature animals, including cattle.
There is a remarkable age-related innate resistance to RVF virus; mortality rate in lambs less than 1 week old exceeds 90% whereas the rate in lambs over 1 week drops to 20%.
The virus is widely distributed in Africa, but major epidemic episodes in animals and humans are relatively rare, occurring in 5 to 20 year cycles. Between epidemics, the virus survives in mosquito eggs laid on vegetation in dambos - which are shallow depressions in forest edges. Only when these are flooded do the eggs hatch. This only occurs when the water table rises following prolonged heavy rain. The eggs then hatch and a new population of infected mosquitos emerges.
 |
| Widespread flooding causing mosquito eggs to hatch |
| © F. Glyn Davies |
Epidemics in domesticated animals are started by the bites of infected mosquitoes. After this infected air generated by virus-infected aborted foetuses and fluids spread the disease rapidly through the flock or herd.
Man is usually infected by breathing route by handling infected animals or tissues. Until recently the disease in man was considered to be a non-serious influenza type illness, but a fatal haemorrhagic form has emerged and now RVF in man is considered to be one of the most dangerous diseases known.
The virus may spread by windborne mosquitoes or by the introduction of infected animals.
Signs of Rift Valley Fever
- The incubation period in lambs is 12 to 36 hours. A fever of up to 41°C (106°F) may develop.
- Peracute infections occur in newborn lambs which die within hours of infection. Acute reactions occur in older lambs and calves and occasionally in adult sheep.
- In very severe infection in calves, death may occur in 2 days after infection without their showing any clinical signs.
- A haemorrhagic syndrome was observed during the last outbreak in Kenya, affecting adult cattle.
- In its severe form, calves will develop high fever, and may vomit. Some nasal discharge may also be seen followed by prostration and mortality may reach up to 70%.
- In mature animals most, if not all, infected pregnant sheep, cattle and camels abort affected foetuses.
- Subacute reactions occur in adult sheep, cattle and camels. There is a low-grade fever, partial lack of appetite and general weakness. Jaundice is prominent.
- In pregnant ewes the mortality and abortion rates varies from 5 - 100%.
Diagnosis
The main post mortem lesion is focal necrosis of the liver but in young animals the foci coalesce to form a diffuse necrotic lesion. The liver is a bright yellow and studded with subcapsular haemorrhages. It is not enlarged. In adult affected sheep and cattle the focal necrosis of the liver is discrete. In addition to the hepatitis there are haemorrhages in most other organs and tissues. Virus isolation from aborted foetuses and from affected animals and serological tests can be used to read a diagnosis.
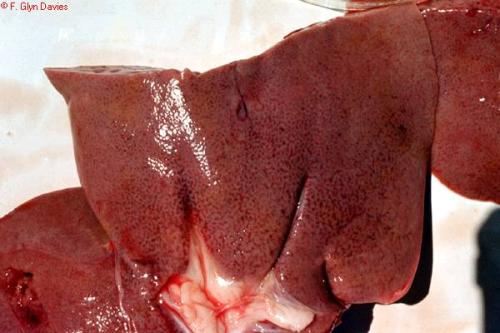 |
| Necrosis of the liver from Rift Valley Fever |
| © F. Glyn Davies |
Recovery from RVF is followed by lifelong immunity.
Prevention - Control - Treatment
Prevention and Control
- Outbreaks of Rift Valley Fever generally occur only after periods of prolonged heavy rain. Such being the case livestock owners should be aware that if long rains are excessively heavy and continue for an excessively long period of time, an outbreak of Rift Valley Fever is likely to occur and action in the form of vaccination is required.
- Control of mosquitoes, movement of stock to higher altitudes and confinement of stock to insectproof stables are usually not practical, instituted too late and of little value.
- Use of pour-on's may well be beneficial and should be used.
- Immunisation remains the only effective way to protect livestock.
- Vaccination of animals with suitable vaccines should be practiced. However live vaccine should NOT be used in pregnant animals.
- Pregnant cows should be vaccinated with killed vaccines to avoid the risk of abortion.
- Humans at particular risk should be vaccinated with formalin killed tissue culture vaccine.
Treatment
There is no known medical treatment for Rift Valley Fever.
Trypanosomiasis / Nagana
Local Names: Luo: tuo maugo, nyalolwe / Kamba: kamosu, ksiko / Kipsigis: kanyagat / Meru: mutombo / Gabbra: ghandi, ndukan, kando / Samburu: itikana, saar / Swahili: lotorobwo, ndorobo /Somali: agku, aino, angsulleh, attech, bargerish, dorobo, dukan, gandi, gindi, gundho, korbarar, malale, salaf, suuilleh / Turkana: edeke lo eidiit, lokipi, lotorob, tikana, lonyang / Maasai: Kububwuo, dorobo / English: Trypanosomosis/Trypanosomiasis
Common Names: Nagana, Surra, Dourine, Sleeping sickness
Description: Fly borne disease
Introduction
Trypanosomiasis is a protozoal infection of cattle, camels, pigs, donkeys, goats, sheep, horses and dogs. It also affects humans as Sleeping Sickness. The disease occurs world wide in tropical and sub tropical countries. It is caused by protozoa of the genus Trypanasoma. The major species are T. congolense, T. vivax, T. brucei, T. evansi and T. simiae.
In Keny trypanosomiasis occurs where both host, parasite and transmitting insects occur. These areas include areas near Lake Victoria, Southern Kenya around Lake Magadi and the Nguruman Escarpment and the Mara, the Coastal areas and around Tsavo, Northern Kenya, parts of the Laikipia Plateau, north of Lack Baringo and along the Tana River. Large areas of Northern Kenya are affected by T. evansi in camels. The cool Highlands are free of disease, except when infected animals are brought in from endemic areas.
Mode of Spread
|
The disease is transmitted in Africa cyclically by Glossina tsetse flies and mechanically by biting flies. Tsetse-transmitted trypanosomes are normally maintained in wild animals. Adult flies derive all their nutritional requirements from vertebrate blood and seek out suitable hosts in daylight by sight and smell. Different specieis of fly favour certain hosts. Warthog, eland, buffalo bushbuck, bushpig, duiker, certain reptiles and hippo are peculiarly sought out by certain flies. In these wild animals the disease is mild and symptomless and they act as reservoir hosts from which domesticated livestock can be infected. |
Animals are infected with trypansomes by the bite of an infected tsetse fly. Tsetse flies themselves become infected when they feed on an infected animal host: the ingested trypanosomes undergo a cycle of development in the fly lasting between 8 to 35 days before infective trypanosomes are produced. Once infected a fly is usually capable of transmitting trypanosomes for the rest of its life. Infection rates in wild populations of tsetse flies are normally low, varying from 1 to 20%.
All species of domestic animals are susceptible to infection but infections are of most importance in cattle. Certain breeds of small humpless West African breeds of cattle i.e. the Ndama are less susceptible to infection than humped Zebu breeds.
Types of Tsetse Flies
Tsetse flies can be divided into three groups - forest, riverine and savannah. Trypanosomiasis becomes important when man and domestic livestock compete with wildlife and tsetse for graying land, or come into contact with tsetse as a result of other activities.
Forest tsetse are of least importance as their habitat is frequently unsuitable for raising livesteock. Riverine tsetse are more important because they infest vegetation near essential water supplies. The most important are the savannah tsetse as the inhabit vast areas of land otherwise suitable for grazing animals. The flies prefer shade and have a limited flight distance. They tend to live in luggas, areas of bush and clumps of trees. From here they venture out to feed on wild and domestic animals.
| Biting flies are involved mainly in the transmission of T. evansi in camels which is transmitted by tabanid camel flies.
Tabanid flies are persistent, aggressive feeders, annoying the host so that feeding is often interrupted, thus ensuring that several new hosts may be bitten, increasing the possibility of multiple infections. Wildlife normally plays little or no part in the transmission of trypanosomiasis in camels. |
|
Signs of Trypanosomiasis
Trypanosomes appear in the blood of infected animals from between 3 to 16 days after initial exposure (picture on title), the time factor being dependant on the species of trypanosome. Trypanosomes vary in their grade of attack. Vivax can cause a severe, rapidly deadly disease with a characteristic haemorrhagic syndrome, brucei can be quite mild, while congolense varies in severity. Mixed infections can occur.
The primary clinical signs are periodic fever, anaemia, and weight loss. In cattle it is a long-lasting (chronic) disease with a high mortality. Rapid onset short but severe (acute) infections occasionally occur, notably with T. vivax in cattle.
- Infected animals are usually tired and weak and are left behind by the rest of the herd. There is an intermittent fever. They have rough, dull coats, progressive anaemia and lose condition.
- Superficial lymph nodes are usually enlarged and prominent, sometimes leading to a mistaken diagnosis of ECF.
- The animal has a foul smell that herders recognize.
- There is a drop in milk production.
- There is a watery discharge from the eyes of sick animals, especially those infected with T. vivax, and the eyes of such animals are cloudy and blink a lot.
- Paleness of the gums, under the tongue and inside the eyes after several weeks of infection.
- Post mortem examination of animals which have died of acute trypanosomiasis may show extensive small haemorrhages involving mucous and serous surfaces, areas of emphysema in the lungs and mild gastroenteritis. After more chronic infections, the carcass may be anaemic and emaciated, with an enlarged spleen and lymph nodes.
- Pregnant animals abort or give birth to weak offspring while some animals become infertile.
The disease is made more severe by factors like poor nutrition, stress and over working. Some animals recover very slowly without treatment while others become very sick, collapse and die after a few months.
Diagnosis
The area, the presence of tsetse flies or, in camel areas, the presence of biting flies, the appearance of weak, anaemic, tired, emaciated animals should be a warning sign that they may be infected with trypanosomiasis. Skilled workers check for trypanosomes in wet blood film by placing a drop of the blood on a slide or by converting the wet film into a smear. The smear is then stained using Giemsa and examined under oil immersion.
The use of small hand-held battery-powered centrifuges has helped greatly in diagnosis in the field. Other diseases can be confused superficially with trypanosomiasis, so demonstration of the parasite is most important. Hence the alerting of a skilled veterinarian is vital to obtain a diagnosis. ECF in acute cases, and worm infestations and malnutrition in chronic cases can be confused with trypanosmiasis.
Prevention - Control - Treatment
Prevention and Control
- Avoid fly-infested areas and minimize contact between livestock and wild animals.
- The use of insecticidal attractants in tsetse traps has considerably reduced tsetse fly numbers in the areas which they have been used, utilising the fact that tsetse flies are attracted to colours such as dark blue. These traps should be used wherever possible. Trapping technology is relatively simple and less polluting to the environment than insecticide application.
- Take animals to water when fly activity is low.
- Repel flies with smoke from smouldering cow dung.
- Seperate sick animals from healthy ones. The disease is not contagious but when cattle are together they attract flies, which may transmit infection.
- Use of trypano-tolerant breeds of cattle such as N'dama and Orma Boran.
- Clear bush to reduce tsetse habitat.
- Spray insecticide on fly breeding areas.
- Use insecticidal pour-ons such as Cypertick and Ectomin.
- Frequent spraying and dipping of animals.
- Use preventative drugs such as Samorin and Suramin.
- Use of trypanocidals for treatment and prevention, and good nutrition, especially where exotic breeds are reared.
Tsetse Trap
Insect specialists at ICIPE found out that tsetse flies are attracted to blue colour, prefer black cloth to land on and will use white colour as an orientation. According to this research findings, traps have been designed with blue/black/white cloth. To discourage theft, slits can be made in the blue cloth. To incease trap efficiency, cow urine is placed under the traps in plastic bottles.
Trap densities: In the case of savannah species each fly may disperse up to 500 metres in a single day, so that with an average trap density of just four traps per km2, there is a high likelihood that each fly encounters at least one trap. However, species living in forests disperse little more than 5-10m per day, so that effective trap densities need to be very much higher.
Traps are relatively inexpensive and lend themselves to community participation. However, problems are experienced due to trap theft, vandalism, and damage by wildlife. To reduce theft of traps, and vandalism requires at the very least a high degree of community education and awareness-raising. Such community awareness can be extended further to community participation, involvement of local people in control activities, and even community-based systems, such as management and financing.
More information on different models of tsetse traps available under www.vestergaard-frandsen.com.
Recommended Treatment
No new drugs for either treatment or prevention have been developed for a very long time so it is vital to use those few that are available properly to avoid drug resistance. Most importantly animal bodyweights should not be underestimated. The following treatments can be used:
- Berenil, Norotryp, etc (Diminazine aceturate): other names Trypazen, Diminazen. Should be prepared by mixing 2.36g of powder with 12.5ml of clean water. The mixture should be given as injection at 3.5mg/kg body weight in the muscle. This amounts to one sachet per 300 kgs bodyweight.
- Novidium (Homidium chloride or bromide): other trade name Ethidium. Can be prepared by mixing 1 tablet 250mg in 10 litre of sterile clean water and used soon after mixing at 1mg/kg body weight injected deep into the muscle.
- Samorin or Trypamidium (Isometamidium chloride): other trade names Trypacide. It can be prepared by mixing 125mg of powder in 12.5ml of clean sterile water and ensuring that all the powder dissolves. Inject the mixture deep intramuscular injection into the neck or slowly intravenously and ensure that medicine goes into the vein. The mixture should be used within 2 days.
- Samorin can be used to prevent the disease, given at intervals of about 4 months. Check dose rate and give according to the correct body weight.
- Camels: Use Tryquin as Berenil can be toxic to camels.
Common Traditional Practices
Traditional practices have been recorded, but no official testing has been carried out to confirm of disapprove the efficacy. Therefore it is highly recommended for all communities to use the methods of control that have been tested and found effective in order to keep their livestock healthy and alive.Review Process
2. Hugh Cran, Practicing Veterinarian Nakuru. March - Oct 2010
3. Review workshop team. Nov 2 - 5, 2010
- For Infonet: Anne, Dr Hugh Cran
- For KARI: Dr Mario Younan KARI/KASAL, William Ayako - Animal scientist, KARI Naivasha
- For Department of Veterinary Services: Dr Josphat Muema - District Veterinary Officer Isiolo, Dr Charity Nguyo - Kabete Extention Division, Mr Patrick Muthui -. Senior Livestock Health Assistant Isiolo, Ms Emmah Njeri Njoroge - Senior Livestock Health Assistant Machakos
- Pastoralists: Dr Ezra Saitoti Kotonto - Private practitioner, Abdi Gollo H.O.D. Segera Ranch
- Farmers: Benson Chenge Kuria and Francis Maina Gilgil and John Mutisya Machakos
- Language and format: Carol Gachiengo
Information Source Links
- Animal Diseases in the Tropics 4th Edition Edited by Sewell and Brocklesby
- Barber, J., Wood, D.J. (1976) Livestock management for East Africa: Edwar Arnold (Publishers) Ltd 25 Hill Street London WIX 8LL
- Blood, D-C.,Radostits, O.M. and Henderson, J.A. (1983) Veterinary Medicine - A textbook of the Diseases of Cattle, Sheep, Goats and Horses. Sixth Edition - Bailliere Tindall London. ISBN: 0702012866
- Blowey, R.W. (1986). A Veterinary book for dairy farmers: Farming press limited Wharfedale road, Ipswich, Suffolk IPI 4LG
- Force, B. (1999). Where there is no Vet. CTA, Wageningen, The Netherlands. ISBN 978-0333-58899-4.
- Hall, H.T.B. (1985). Diseases and parasites of Livestock in the tropics. Second Edition. Longman Group UK. ISBN 0582775140
- Hunter, A. (1996). Animal health: General principles. Volume 1 (Tropical Agriculturalist) - Macmillan Education Press. ISBN: 0333612027
- Hunter, A. (1996). Animal health: Specific Diseases. Volume 2 (Tropical Agriculturalist) - Macmillan Education Press. ISBN: 0-333-57360-9
- ITDG and IIRR (1996), Ethnoveterinary medicine in Kenya: A field manual of traditional animal health care practicies. Intermediate Technology Development Group and International Institute of Rural Reconstruction, Nairobi, Kenya. ISBN 9966-9606-2-7
- Pagot, J. (1992). Animal Production in the Tropics and Subtropics. MacMillan Education Limited London.
- The Merck Veterinary Manual 9th Edition, Merck & Co Inc Whitehouse Station NJ USA
- The Organic Farmer magazine No. 50 July 2009
- The Organic Farmer magazine No. 51 August 2009
- Vestergaard Frandsen (Supplier of tsetse traps and other technologies for disease control in the tropics) www.vestergaard-frandsen.com.
 Back
Back


