Phosphorus (Environmental & Earth Science)
Contents
Phosphorus
Phosphorus is an element with the symbol P and of atomic number fifteen. This non-metallic element belongs to group VA of the third period of the Periodic Table. Phosphorus in the Earth's crust generally occurs in its maximally oxidized state, such as inorganic phosphate rocks. Elemental phosphorus is found in three major versions: White, red and black phosphorus, but because of its extreme chemical reactivity, phosphorus does not exist as a free element on Earth.
| Previous Element: Silicon Next Element: Sulfur |
| |
| Physical Properties | ||
|---|---|---|
| Color | white-yellow | |
| Phase at Room Temp. | solid | |
| Density (g/cm3) | 1.823 | |
| Hardness (Mohs) | 0.5 | |
|
Melting Point (K) |
317.3 | |
|
Boiling Point (K) |
553.7 | |
| Heat of Fusion (kJ/mol) | 2.5 | |
| Heat of Vaporization (kJ/mol) | --- | |
| Heat of Atomization (kJ/mol) | 315 | |
| Thermal Conductivity (J/m sec K) | 0.24 | |
| Electrical Conductivity (1/mohm cm) | 0 | |
| Source | Apatite | |
| Atomic Properties | ||
| Electron Configuration | [Ne]3s23p3 | |
|
Number of Isotopes |
23 (1 natural) | |
| Electron Affinity (kJ/mol) | 72.07 | |
| First Ionization Energy (kJ/mol) | 1011.7 | |
| Second Ionization Energy (kJ/mol) | 1903.2 | |
| Third Ionization Energy (kJ/mol) | 2911.9 | |
| Electronegativity | 2.19 | |
| Polarizability (Å3) | 3.6 | |
| Atomic Weight | 30.974 | |
| Atomic Volume (cm3/mol) | 17 | |
| Ionic Radius2- (pm) | --- | |
| Ionic Radius1- (pm) | --- | |
| Atomic Radius (pm) | 110 | |
| Ionic Radius1+ (pm) | --- | |
| Ionic Radius2+ (pm) | --- | |
| Ionic Radius3+ (pm) | 58 | |
| Common Oxidation Numbers | -3, +3, +5 | |
| Other Oxidation Numbers | -2, -1, +1, +2, +4 | |
Phosphorus is a critical element in all lifeforms on Earth, firstly due to its essential occurrence in all DNA or nucleic acids that carry organism genetic code. Secondarily it is present in adenosine triphosphate molecules as well as the phospholipids that are intrinsic in all cell membranes. Phosphate is an essential plant nutrient, but excessive levels of phosphate in water systems lead to water pollution, algal blooms and aquatic ecosystem imbalances.
Phosphorus Cycle
The phosphorus cycle is a biogeochemical cycle that describes the movement and chemical transformation of phosphorus through the geosphere, hydrosphere and biosphere. Unlike many other biogeochemical cycles, the atmosphere does not play a significant role in the movement of phosphorus because phosphorus and phosphorus-based compounds are usually solids at the typical ranges of temperature and pressure found on Earth. Therefore most of the phosphorus remains within rock, sediments, sand, and the ocean floor and within a fraction in living matter.
Natural Occurrence
Substantial amounts of phosphate are mined from the Earth's crust, with a disproportionate quantity being derived from central and north-central Florida, which locale has historically produced about three fourths of the world supply of phosphate rock. The Florida phosphate deposits are also rich in Uranium content, which may create health issues for instances where these phosphates are used in crops capable of high uptake rates for Uranium; tobacco is one such planttype, implicating radioactivity dosage to human lung tissue as a contributory cause of lung cancer.
Most of the world's known reserves are found in the Middle East, North Africa, China, Russia and the USA; there are widely ranging estimates of the exhaustion of phosphate reserves, ranging from 30 to 300 years. Besides China's own reserves, that country effectively controls much of North Africa's ore through long term lease agreements.
Allotropes
Phosphorus has several allotropes, the two most common being termed white and red phosphorus. White phosphorus is a highly toxic, waxy looking, white substance. Its molecular structure is tetratomic, with four phosphorus atoms at the corners of the tetrahedron. this tetraphosphorus allotrope is highly reactive due to its strained bond angles. When the tetraphosphorus is isolated in the laboratory, a chemical reaction occurs upon exposure to oxygen, producing short-lived molecules of HPO and P2O2 that emit visible light as they de-excite from an elevated state of electronic excitation. In fact, white phosphorus spontaneously combusts in air above 30 degrees Celsius. Since the combustion reaction itself proceeds slowly, the luminescence period is prolonged. This white phosphorus allotrope has a melting point of 44 degrees Celsius. White phosphorus is so reactive that it must be stored under water, in which it is highly unsoluble and minimally reactive; conversely, tetraphosphorus is extremely soluble in non-polar organic liquids.
 White phosphorus sample with corner sliced. For the red phosporus allotrope, one phosphorus-phosphorus bond is broken, and an external bond is created with a nearby tetrahedron, producing a somewhat concatenated macromolecule. Red phosphorus is produced from the tetraphosphorus allotrope upon heating to 250 degrees Celsius or exposure to bright sunlight. Thus red phosphorus is not a well defined molecule, but rather an assortment of long chain molecules. Black phosphorus is formed from similar concatenated chains, but the resutting structure is more regular and graphite-like, yielding a form stable to high temperatures.
White phosphorus sample with corner sliced. For the red phosporus allotrope, one phosphorus-phosphorus bond is broken, and an external bond is created with a nearby tetrahedron, producing a somewhat concatenated macromolecule. Red phosphorus is produced from the tetraphosphorus allotrope upon heating to 250 degrees Celsius or exposure to bright sunlight. Thus red phosphorus is not a well defined molecule, but rather an assortment of long chain molecules. Black phosphorus is formed from similar concatenated chains, but the resutting structure is more regular and graphite-like, yielding a form stable to high temperatures.
Phosphorus Chemistry
Halides
The trihalides phosphorus trifluoride, PF3, phosphorus trichloride, PC3, phosphorus tribromide, PBr3 and phosphorus triiodide, P3 and the pentahalides, phosphorus pentachloride PCl5 and phosphorus pentabromide, PBr5 are prevalently used in phosphorus chemistry; furthermore, mixed halides may also be synthesized; however, the mixed halides are inherently unstable and decompose to form simple halides Pentahalides; For example, 5PF3Br2 decomposes into 3PF5 and 2PBr5. Phosphorus pentahalides are synthesized by combining excess halogen with either elemental phosphorus or with the corresponding trihalide. .
Oxides and Oxyacids
Phosphorus trioxide, P4O6, and phosphorus pentoxide, P4O10 are acid anhydrides of phosphorus oxyacids and thus readily react with water. P4O10 is a particularly effective dehydrating agent capable of removing water from nitric acid. The structure of P4O6 resembles that of P4 with an oxygen atom inserted between each of the phosphorus-phosphorus bonds. The molecular design of P4O10 is like that of P4O6 with the addition of one oxygen bond to each phosphorus atom via a double bond, extending away from the tetrahedron.
Phosphorous oxyacids may exhibit acidic protons bound to oxygen atoms or nonacidic protons that are bound explicitly to the phosphorus atom. Although there are numerous phosphorus oxyacids, merely six are significant, and three of them, hypophosphorous acid, phosphorous acid and phosphoric acid are particularly key.
Phosphines
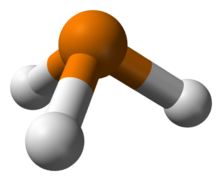 Phosphine stick and ball model molecule. Phosphine (PH3) is a periodic table analogue of ammonia, forming a pyramidal structure with the central phosphorus atom bound to three hydrogen atoms. Both phosphine and ammonia are colourless, ill-smelling, toxic compounds. Phosphine is unstable and reacts at once with air yielding phosphoric acid clouds. Phosphine can form certain phosphonium salt such as PH4CI, analogues of ammonium salts, but such salts immediately decompose in water and do not yield phosphonium (PH4+) ions. Diphosphine (P2H4 or H2P-PH2) is a colourless liquid that spontaneously ignites in air and can yield phosphine and complex hydrides.
Phosphine stick and ball model molecule. Phosphine (PH3) is a periodic table analogue of ammonia, forming a pyramidal structure with the central phosphorus atom bound to three hydrogen atoms. Both phosphine and ammonia are colourless, ill-smelling, toxic compounds. Phosphine is unstable and reacts at once with air yielding phosphoric acid clouds. Phosphine can form certain phosphonium salt such as PH4CI, analogues of ammonium salts, but such salts immediately decompose in water and do not yield phosphonium (PH4+) ions. Diphosphine (P2H4 or H2P-PH2) is a colourless liquid that spontaneously ignites in air and can yield phosphine and complex hydrides.
Isotopes
There are 23 known phosphorus isotopes; the 31P isotope with spin one-half is the sole stable version, and is thus present at 100 percent abundance. The half-integer spin and abundance of 31P make it a convenient research tool for tagging and investigation of biomolecules, particularly DNA. Two radioactive isotopes of phosphorus have half-lives that make them especially useful for scientific experiments. 32P has a half-life of 14.262 days and 33P has a half-life of 25.34 days.
Health Effects of White Phosphorus
Contact with pure white phosphorus is unlikely, due to the extremely high reactivity of this substance leading to rapid conversion of white phosphorus to typically less harmful compounds; therefore, the incidence of health effects is generally rather low.
Inhaling pure white phosphorus can cause coughing or developing a condition that involves poor wound healing in the mouth and a breakdown of the jaw bone. Damage to the blood vessels of the mouth has been observed in rats breathing air containing white phosphorus. Ingestion of small amounts of white phosphorus (less than one teaspoon), may cause vomitting, stomach cramps, or lead to liver, heart or kidney damage. Because of the lack of cancer studies on animals or people, the EPA has determined that white phosphorus is not classifiable as to human carcinogenicity (that is, whether or not it causes cancer).
References
- Geoffrey Rayner-Canham and Tina Overton. 2003. Descriptive inorganic chemistry. Macmillan. 569 pages
- John Emsley. 2000. The Shocking history of Phosphorus. A biography of the Devil's Element. London: MacMillan
- G.M.Filippelli. 2002. The Global Phosphorus Cycle. Reviews in Mineralogy and geochemistry 48: 391–425
- B.A.Kennedy and Bruce A.Kennedy. 1990. Surface mining. Society for Mining, Metallurgy and Exploration. 1194 pages
- J.Roels and W.Verstraete. 2001. Biological formation of volatile phosphorus compounds. Bioresource Technology 79
- Arndt Simon, Horst Borrmann and Jörg Horakh. 1997. On the Polymorphism of White Phosphorus. Chemische Berichte 130: 1235.
- A.P.Sincero and G.A.Sincero. 2002. Physical-chemical treatment of water and wastewater. IWA Publishers. 784 pages.
