Nanotechnology standards: an issues landscape
| Topics: |
This content is not assigned to a topic
|
In September 2006, the Institute for Food and Agricultural Standards (IFAS, now called the Center for the Study of Standards in Society [CS3]) at Michigan State University hosted a workshop on the development of standards for emerging nanotechnologies. Participants were drawn from university, corporate, government, and non-governmental sectors, representing a wide range of expertise and perspectives. This report is the outcome of two days of discussion, which identified major features of the nanotechnology standards landscape. The purpose of this report is to memorialize the perspectives of the participants in the development of this evolving technology. As such, this document reflects the thinking of a broad cross section of nanotechnology stakeholders at this moment in time.
Contents
- 1 Executive Summary
- 2 Introduction
- 3 Timing and Standards-Setting
- 4 Product Versus Process Standards
- 5 International Harmonization
- 6 Integration of Operational Standards
- 7 Participation and Transparency in Standards-Setting
- 7.1 Defining and Operationalizing the Concept of Standards
- 7.2 Define and Identify Potentially Affected Groups
- 7.3 Preferred Models of Participation
- 7.4 Public Meetings are Insufficient
- 7.5 Drivers of Information/Insight Gained through Public Participation
- 7.6 Risk Communication
- 7.7 Limits of/to Participation
- 7.8 Toward Greater Transparency in Standards-setting Processes
- 8 Conclusions
- 9 Acknowledgments
- 10 Notes
- 11 Sources for standards and nanotechnology
Executive Summary
Research, development and commercialization of nanotechnologies are moving forward in a period of uncertainty about elaboration of standards and regulation. This uncertainty poses both opportunities and constraints for a variety of stakeholder interests across different sectors of economic activity. Basic research is needed to determine the health, safety and environmental impact of emerging nanotechnologies so that standards-setting and regulatory processes can keep pace. Common nomenclature and cooperative frameworks should be established early in the process of technology (Clean Technology) development. This report aims to identify key workshop issues from a variety of stakeholder perspectives; these issues point to areas of inquiry for standards development that workshop participants believed to be the most pressing. Five landmarks, or themes, were used to organize the workshop discussion and comprise this issues landscape.
Timing and Standards-Setting
Standards and regulations for nanotechnology need to be developed for all of the stages in the life cycle of the products (research, production, products, waste (Technology and the materials cycle), etc.). Research into nanotechnology is already moving forward under existing rules for lab safety, but development of nanotechnology-specific standards is needed. The production phase is also likely to be a high-risk point. Agencies experienced with worker health and safety should, therefore, be engaged early. Moreover, standards and regulations for nanotechnology use in consumer products are also lacking, and there is disagreement about whether new legislative authority is needed to guide the elaboration and implementation of such efforts. Finally, standards, guidelines and regulations for nanotechnology lab waste, production waste, and end-of-product-life waste remain open questions. Both private and governmental actors should collaborate to address these issues. These areas should be prioritized, based on the most current safety and risk data, and adequately funded for risk analysis to ensure that standards-setting is able to keep pace with research and development.
Product vs. Process Standards
Nanotechnology raises questions about where within the life cycle of a product it makes most sense to place various standards and regulations. Research on nanotechnology risk assessment and analysis will be useful in determining the most efficacious way to implement these standards and regulations. Different government agencies have different mandates in this regard and this will likely have a large impact on whether product or process standards will be developed in any given product or sector. Interagency cooperation is necessary to create the most effective standards. Agreement is needed on the goals of the standards to clearly decide whether it makes more sense to regulate products or processes.
International Harmonization
The US, EU and Japan are all investing significantly in nanotechnology development. Given the global economy, it is certain that intermediate nanotechnology products and finished goods will be marketed globally. This calls for at least limited international harmonization of standards and regulations. Dialogue and cooperation among diverse stakeholders is needed to determine which standards should be harmonized and what the commitments to international enforcement should be made. Ultimately, the debate over international harmonization of standards for any technology is a debate about national difference in approaches to worker health and safety, environmental protection, Economic competitiveness, etc. A certain level of national autonomy in these realms is reasonable.
Integration of Operational Standards
The development of effective nanotechnology government regulations will require that the several agencies that have not historically worked together begin to do so. Mechanisms for interagency cooperation should be primary goals in this process. Achieving information sharing and effective interagency communication will serve as first steps towards more effective action. Lawmakers can assist in this process by providing adequate funding and clear authority to integrate agency mandates. Careful consideration is needed, however, in choosing an appropriate model. Top-down models should be avoided, and promising bottom:up models explored. The Coordinated Framework for Biotechnology[1] also provides a possible, though limited, initial model. Where appropriate, integration with the private sector is also recommended, as some standards developing organizations may act as de facto standards-setting bodies without requiring specific regulatory agency action. ISO, Codex, and the IPPC offer good models for this kind of integrative approach.
Participation and Transparency in Standards-Setting Processes
It was once seen as acceptable for subject experts, government agencies and business interests to debate and establish standardswith little or no input from the larger public. This approach has justifiably been called into question in recent years. In the future, stakeholders should expect to see more attempts at public participation in the standards- setting process than has previously been the case. The type and nature of public participation is largely undefined, and it is this area that needs most attention. For public participation to be seen as legitimate in the eyes of the public, careful attention needs to be paid to identifying potentially affected groups and engaging them in meaningful ways in the [[standards]-setting process]. Several possible models for making this a reality were discussed. Standards-setting bodies need to review and learn from models that have been more successful than the typical “public meetings” model common in the US regulatory system.
The purpose of the standards workshop and this report is not to establish consensus around these themes, but rather to chart the ‘issues landscape’ facing the nanotechnology standards and regulatory communities. As such, this report serves as a roadmap to inform the standards deliberations of agencies and organizations confronting emerging nanotechnologies and their potential applications both within and across different sectors of economic activity.
Introduction
The new nanotechnologies have been singled out by their proponents as unique. Their very uniqueness poses problems for standards. Even the development of the nomenclature necessary for the description of the new nanotechnologies is a complex task currently being undertaken by several organizations (e.g., International Organization for Standardization [ISO] through the American National Standards Institute [ANSI]). There is little data available to inform standards for the health and safety of nano-materials. For instance, Maximum Residue Levels have yet to be established.
Moreover, as a group, nanotechnologies offer a wide variety of challenges and opportunities. Historically, much standard-setting has been reactive in response to injuries, while current efforts involve trying to be more proactive. This suggests a two-pronged approach: On the one hand, we may need reactive standards, i.e., standards for reporting negative incidents. These might be similar to those currently used in the food industry to report food safety problems. On the other hand, anticipatory standards are probably more desirable. However, these would almost undoubtedly have to be linked to particular products, since without a consensus on the product it is extremely difficult to develop an effective standard.
In the US context, the term ‘standards’ is often applied to both voluntary standards set by various private and non-profit organizations as well as to mandatory public regulations set by government agencies. In contrast, in the EU voluntary standards are usually contrasted with government regulations. However, in recent years, in part as a result of increased global trade, the distinction between standards and regulations has become blurred. Many nominally voluntary standards have eventually become de facto mandatory government regulations. In this document we follow the US usage, which was employed during the workshop, distinguishing where appropriate for readers, between voluntary standards and mandatory regulations
In the United States, attempts to coordinate federal work on the nanoscale began in November 1996, when staff members from several agencies held formal meetings under the auspices of the National Science and Technology Council to develop and coordinate plans in this area (Stone and Wolfe 2006). In 2001, the Clinton administration raised nanoscale science and technology (Clean Technology) to the level of a federal initiative, officially referring to it as the National Nanotechnology Initiative (NNI). The NNI now coordinates the multiagency efforts in nanoscale science, engineering (National Center for Science and Engineering Statistics), and technology under the ‘21st Century Nanotechnology Research and Development Act’ (108 P.L. 153, 2003). Twenty-three federal agencies presently participate in the NNI, eleven of which have research and development (R&D) budgets for nanotechnology. Other federal organizations contribute studies of the applications from those agencies performing R&D, as well as other collaborations. The ‘Supplement to the President’s 2006 Budget’ (National Nanotechnology Coordination Office [NNCO] 2005) recommends overall NNI investments for 2005-06 of about $1.05 billion, with $82 million devoted to ‘ Societal Dimensions’ including ‘Environmental, Health, and Safety R&D’ ($38.5 million) and ‘Education and Ethical, Legal, and Other Societal Issues’ ($42.6 million). As one of the agencies participating in the NNI, the National Science Foundation (NSF) sponsors a number of nano-related priority areas. For example, NSF’s Nanoscale Exploratory Research, Nanoscale Interdisciplinary Research Teams, and Nanoscale Science and Engineering Centers fit within its Nanoscale Science and Engineering initiative. In fiscal year 2005, total funding for these NSF programs exceeded $296 million. Recent government projections suggest that funding for nanotechnology will continue to rise across all sectors, with global expenditures projected to exceed $1 trillion by 2015 (Roco 2003).
NSF is the major source of federal funding for research related to nanoscience and nanotechnologies. The Agrifood Nanotechnology Project at Michigan State University (MSU), through which the International Nanotechnology Standards Workshop was convened, is supported by an NSF grant to examine social and ethical dimensions of nanotechnologies in the agrifood supply chain.
The Agrifood Nanotechnology Project is jointly conducted through the Department of Community, Agriculture, Recreation and Resource Studies (CARRS) and the Institute for Food and Agricultural Standards (IFAS) (now the Center for the Study of Standards in Society [CS3]) at MSU. The CS3 mission is to raise fundamental issues with respect to equity, fairness and transparency of food (Food) and agricultural (Agriculture) standards at the local, national and international levels.
Standards are generally considered to be convenient, neutral, and benign means for handling issues of technical compatibility without involving formal government actions. However, since social power involves the ability to set the rules that others (must) follow, then standards represent a form of codified power reflecting the interests of those groups with greatest access to and influence within standards-setting processes. While many people and institutions recognize and broadly support the role of standards in general, controversy often ensues as they confront the question: “Whose Standards?.” CS3 recognizes that standards are shaped by cultural, ethical, political and strategic, as well as technical considerations, and this perspective guides the standards-related activities associated with its Agrifood Nanotechnology Project[2].
The CS3 project supports a number of research and outreach activities pertaining to the development of nanotechnology standards. These activities include their potential integration with standards for other technologies as well as with standards regimes operating across specific spheres of economic activity, such as global agrifood [[supply chain]s]. For example, a CS3 project team presently holds a seat on the ANSI Nanotechnology Standards Panel, from which it is able to observe and to some extent participate first-hand in the standards facilitation process. The Agrifood Nanotechnology Project has also sponsored a series of nanotechnology conferences and workshops, of which the standards workshop reported here is a part. The first of these, convened in October 2005, examined experience with agrifood biotechnologies as seen from a variety of stakeholder perspectives – essentially interrogating the lessons learned from this prior experience to inform the ongoing development of nanotechnologies (David and Thompson 2008).[3] Of the many lessons learned, perhaps the greatest consensus centered on the perceived failure to engage diverse stakeholders and other potentially affected groups in a dialogue as standards for agricultural biotechnologies were being promulgated. Conference participants agreed that an early dialogue among diverse stakeholder interests should precede the development of standards for emerging nanotechnologies, so as to better identify the social landscape and potential distributive consequences of standards decisions.
The international nanotechnology standards workshop reported herein was in large part a response to these lessons learned. The workshop was convened to bridge the interests of a variety of communities that might not normally communicate with each other on standards issues, but which nonetheless maintain a mutual interest in them. The goals of the workshop reported here were to:
- Stimulate public discussion and understanding of issues involved in developing nanotechnology standards,
- Influence public and private agendas with respect to nanotechnologies, and
- Link diverse and distinct communities concerned with nanotechnologies.
Although the workshop addressed standards and regulatory issues likely to be relevant for the agrifood sector, emerging nanotechnologies are expected to cut across numerous sectors, potentially blurring traditional boundaries. Thus, workshop participants included representatives from a wide variety of perspectives, including business and industry, government regulatory agencies, labor groups, non-governmental organizations, [[trade] associations], and standard-setting bodies, as well as numerous academic and technical disciplines both domestically and internationally.
Prior to the workshop, the Agrifood Nanotechnology Project created a web forum for participants and interested invitees to begin online discussion on each of five critical standards themes. Thereby they were able to shape the workshop agenda more carefully and ensure that it addressed their concerns. The five themes were:
- Timing and Standards-Setting
- Product vs. Process Standards
- International Harmonization
- Integration of Operational Standards
- Participation and Transparency (Sustainable Society Index)in Standards-Setting Processes
After opening the workshop, participants were divided into five small working groups so as to facilitate discussion. To maximize variation of perspectives, each breakout group consisted of members from each of the stakeholder categories identified above. These groups met in separate rooms during the workshop, where they identified and debated key questions and issues surrounding each of the five critical standards themes. Project team members were assigned as facilitators and note-takers for each of the groups. Each breakout session lasted roughly two hours after which all of the groups reconvened at plenary sessions to report on their deliberations. Following the workshop, the project staff compiled notes from each breakout group and organized them around each of the five standards themes. These notes were drafted into text reflecting key issues and questions within each theme.
Early drafts of this report were posted on the workshop web forum for participant review and comment. This process helped to further clarify key issues and questions.
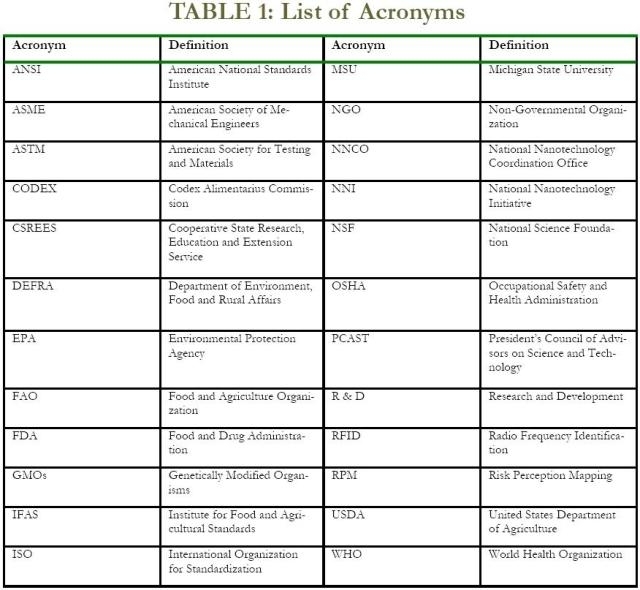
This final document presents a synthesis of ideas focused around each of the five themes. It should be noted that the purpose of this exercise was not to establish consensus around these themes, but rather to chart the ‘issues landscape’ facing the nanotechnology standards communities. As such, it is designed to serve as a roadmap to inform the standards deliberations of agencies and organizations confronting emerging nanotechnologies and their potential applications both within and across different sectors of economic activity. Table 1, below, provides a list of acronyms and associated definitions as used in this document.
Timing and Standards-Setting
The timing of discussion and identification of standards for new technologies is a critical point of dialog among academics, governments, non-governmental organizations, industry, and the general public. With the rapidly emerging field of nanotechnology, it is important to balance the need for free inquiry with the need to protect society through the development of guidelines, whether voluntary standards or government regulations. This includes standards for basic research laboratories, product development laboratories, and manufacturing facilities, as well as environmental, and consumer healthand safety regulations.
Three interrelated themes pertain to the timing of standards-setting, including whether: (1) the guidance-setting process for nanotechnologies should begin early in the knowledge development process, or later as such knowledge is applied to the development of new products and processes; (2) a timeline can be developed that is acceptable to all interested parties; and (3) these issues can be addressed appropriately and strategically with respect to global economic competition. Standards will need to be developed for all aspects of the new nanotechnologies: research, production (Exposure standards and guidelines), products, use, and waste disposal.
Research
Research standards should be informed or modeled on the use of Good Laboratory Practices, as used in food and drug sectors, e.g., special gloves, respirators, and hoods (although even these standards may need further development). For instance, many gloves commonly used today in conventional lab work are ineffective for preventing penetration by nanoparticles. Some companies and universities already employ standards for Good Laboratory Practices as a matter of routine. However, fully eliminating exposure might have the effect of creating a de facto ban on nano materials.
Since university-based research is often conducted further upstream from industrial research, it may require standard procedures that are different from those for industrial research. At the same time, virtually all universities are faced with declining resources for facilities. Institutions should ensure that researchers, post-docs, and students are adequately protected from what remain largely undefined or poorly defined hazards.
Given the scarcity of information about risks associated with nanotechnologies, initial information and insight should be collected based on experiences in the laboratory. This suggests that there is already a need for standards for reporting incidents of concern, providing guidance on what kinds of incidents should be recorded as ‘potential negatives,’ and which incidents should be reported to what agencies. It should be noted, however, that there are already regulations in place for reporting adverse effects to either the EPA (Establishment of the U.S. EPA)(for the chemical industry) or to the FDA(for drugs).
Production
Since the highest risks are likely to be during the production phase, and not in the final products, standards are needed to protect workers from exposure. Even in the production phase, there are means for reducing risk. As risk is a function of exposure and hazard, and we do not yet know precisely what the hazard is, then efforts should be made to reduce exposure. The National Institute for Occupational Safety and Health has a good track record in working with industry in similar situations. Its expertise should be welcomed.
Products
One problem posed by the new nanotechnologies is that every new product seems like a special case. Moreover, different products will fall under the jurisdiction of different regulatory agencies. For example, a product which is overtly therapeutic will fall under the Food and Drug Administration (FDA) approval process and require clinical trials.
Food and drug products will likely need standards as soon as products are developed. In contrast, there is less urgency for non-consumable products, although environmental issues still need to be addressed. Yet, even the determination as to whether a new food or drug product is substantially different requires a standard. Furthermore, there are naturally occurring nanoparticles in our food (Food) and in other consumer goods now. Standards developers will need to differentiate between these naturally occurring nanoparticlesand those that are manufactured.
One issue of considerable importance with respect to products will be public disclosure. Many, if not most, nanotechnology products will involve Confidential Business Information (CBI) during the registration process; therefore, only the commercial applicant’s “robust summaries” (of risk relevant data, for example) if any, will be available to the general public. Currently, no pre-market notifications are required for nanotechnology products, if the larger scale version is already registered. According to the manufacturers, nanotubes are in tennis rackets now. Labeling may pose yet another set of problems.
The insurance industry is likely to have a significant effect on the use of nanotechnologies in consumer products. Currently, insurers are uncertain about their capacity to insure against damages resulting from the production or consumption (Consumption) of products with nano materials due to the lack of standards, and their inability to calculate their actuarial exposure to economic risk. One effect of this uncertainty will be pressure on firms using nanotechnologies to self-regulate in order to avoid tort cases.
Waste Disposal
Once nanoparticles have come together in, for example, a tennis racket, do they come out again? These particles pose a unique end-of-product-life concern. Virtually all industries have environmental discharges, and many engage in wastewater treatment. Some participants maintained that nanoparticles are not differentiated in industrial processes, leaving issues for the separation of waste streams. Moreover, the liquids in which nanoparticles are stored may be demonstrably more toxicthan the particles themselves. However, as others remarked, this may be an overly alarmist concern, noting that existing materials are generally shipped as powders, and waste streams are primarily water. Other liquids might be those the material is shipped in, which would mean it may not be a waste. In either case, it remains unclear whether current Waste treatment procedures and processes are sufficient to filter out nanoparticles.
Given the potential differences in the behavior of chemicals at the nanoscale level, it is conceivable that the Environmental Protection Agency (Origins of the Environmental Protection Agency) will need to re-review every chemical (Regulation of toxic chemicals) in its database. The task will be daunting, and one for which funds are currently lacking. This suggests that standards need to be developed now to prioritize according to toxicity (Toxicity testing methods) and likelihood of use.
Product Versus Process Standards
In many areas of new technology development, debates have arisen as to whether standards should focus on process or on product. For example, standards for organic foods are process standards prescribing particular production processes to be followed in order to meet standards, while those for pesticide residues are product standards defined by the quantity of pesticides remaining in/on the food product at the point of consumption. Similarly, one can distinguish between standards for products bearing nano-engineered materials (e.g., new quality attributes, safety issues, etc) and those for production processes and management systems (how nano-devices are made, and also for nano-enabled processes used to generate new or modify existing products bearing no nano-engineered materials).
Three interrelated themes emerge in this area, including: (1) whether standards for nanotechnology should be primarily product-based or process-based; (2) the extent to which the answer to this question is dependent upon (or likely to vary according to) the specific nanotechnology in question; and (3) whether such standards should vary by the intended application both within and across particular sectors of economic activity (e.g., agrifood, medicine, energy (Energy), security, etc.).
Definition of Product and Process
How these questions are addressed depends on the working definitions of process and product. Process could refer to an engineering (Earth systems engineering and management) process, production (Production possibility frontier) process, or a governance (Global Environmental Governance: Key Challenges to Effective Global Environmental Governance) process. Nanotechnology is such a broad category that pinning down ‘nanotechnology product’ or ‘nanotechnology process’ might be like trying to set a standard for products/processes of biology.
It is similarly difficult to define the product. Is it what the consumer touches, or is it the primary materials, before they are purchased and used by a consumer? Here, a standard might be developed to determine what counts as a nanotechnology process or product, and this could help avoid too imprecise a definition of nanotechnology. This might require the creation of new language and new nomenclature, which would help establish triggers for when nano-standards would be used. Currently, some products might be missed because they are not labeled ‘nano.’
Some processes in nanotechnology might be considered well-established, although their products are novel. It might be much easier to have standards for products, given that there are so many ways to process things. But some processes might be seen as unacceptable, even though the product produced by that process is acceptable. Workers can be subjected to considerable risk, even as the products they produce meet all health (Modern environmental health hazards) and safety standards. Moreover, these concerns are not limited to health and safety. Thus, it is likely that few cases exist where both process and product issues did not both apply.
Some examples seem to blur the process/product line. Organicstandardization can be seen as a touchstone for process standards. Meat standards blur the line, since often the kind of product, e.g., veal, is directly linked to the process. Milk, meat, and egg standards show that both process and product are currently standardized. ‘Kosher’ and ‘Native American’ (e.g., Indian Agriculture Council) similarly embody both process and product standards and tend to blur the process/product distinction.
Risk
The assessment of various nanotechnologies may force a prioritization of risk with respect to nanotechnology standards. Part of this assessment will reveal if, or when, there are special impacts of nanotechnologies. Decisions to address product or process might emerge from the risk assessment of a given nanotechnology. At the limit, addressing issues of risk will require thinking about standards in a manner that is much broader than risk assessment itself. However, there was some question about whether nanotechnologies are processes that are sufficiently unique as to require process standards.
Agency Interaction
Because of the potential blurring of process vs. product standards mentioned above, it may be necessary to identify which agencies might focus more on product standards and which on process standards. For example, the Joint FAO (Food and Agriculture Organization (FAO))/WHO Expert Committee on Food Additives might be asked to assess risks of nanoparticles in foods, or in food packaging. The Organic Crop Improvement Association might be likely to be guided by process standards, whereas EPA and FDA regulate products. These organizations might best start with an existing process or product standards and then extend that to related nanotechnologies, depending on whether the nanotechnology in question is applied during processing or in the final product. However, even if existing standards appear adequate to include new techniques and products, they are rarely applied in practice There is a fear that the appearance of having covered some emerging techniques is only that—an appearance. The Occupational Safety and Health Administration is approaching nanotechnologies with some consternation. There is a widespread myth in the biotechnology (Agricultural biotechnology) debate: that the US only regulates products, and the EU only regulates processes. REACH, for example, is a product-based regulatory framework in the EU. Workshop participants felt this myth should be avoided in agency interactions involving nanotechnologies.
The process/product question raises the issue of how to audit/monitor process standards. Strategies could include third party auditing, accreditation, and certification. Third party is favored for control, but raises concerns about cost and limits on producers’ freedom to operate. Post-market monitoring remains a huge issue.
Decisions regarding whether to regulate by product or process standards may vary by sector of economic activity, reflecting differences in legislative mandates across sectors. For example, it appears that FDA does not have the authority to label by process. Again, the outlier seems to be food and not drugs, devices, cosmetics.
Goal-driven Standards
Part of the difficulty in deciding between process and product standards has to do with the goals of standardization and those of particular nanotechnologies. Is the goal to protect consumers, protect workers, or limit or promote certain kinds of commerce? Is the goal to reassure the public? The public seems more reassured by product-specific standards rather than those related to processes. It can be argued that a product-only standards environment would result in de facto privatization of standards. It is clear that some smaller and startup organizations struggle due to their inability to get access to standards-setting processes. An important divide emerges: On one side is the view that process standards are concerned with values, and that product standards relate (more objectively) to safety or health (Agricultural Health Study). On the other hand, it can be argued that process and product themselves are so linked that one cannot separate value concerns from health/safety concerns.
Furthermore, the Costof standardization will enter into decisions about when, where, and if product or process standards are used. Many supported the view that process standards are harder to monitor and would therefore be more costly, especially in a third party auditing situation. Likely, standards will vary according to the specific nanotechnology in question.
Ordering and Goals of Standards
Standards for nanotechnologies may be approached from three directions:
- Specifications for the production of the nano device, and the engineering practice of producing the nano-product;
- How that nanoproduct is integrated into other production [[supply chain]s];
- How nanotechnologies are integrated into the product itself.
Convergence and Jurisdiction
One issue that is relevant to this question is the blurring of lines between sectors because of nanotechnology’s convergent nature. The processes and products of nanotechnology cross traditional sector lines. One way to deal with this is to stipulate that any relation to nanotechnology require the process or product to be listed as a nanotechnology.
Another variant that may determine the extent to which product/process standards are used is jurisdiction. States and countries will employ different approaches, a strategy generally seen as desirable. For example, Brazilian experience with biotechnology has shown that a lack of negative connotations for nanotechnologies may push some nations to regulate only products, instead of processes (Mattoso, Merdeiros, and Martin-Neto, 2005; Rattner, 2005).
Potential Social Transformations
Some humanists and social scientists argue that the social dynamics of nanotechnologies are qualitatively different than earlier technologies and they have the potential to bring about profound social changes. Doubtless, process/product standards will accompany potential social transformations with respect to goals, regulation and technology (Technology). There are people talking about a “new industrial revolution.” This is a problematic goal, if it merely means that a few people will profit in an unregulated environment.
The philosophical issue of enhancement vs. medical application is a related speculative issue. It is unclear whether some procedures are intended to enhance or treat, solve problems or ‘just’ improve things in a more cosmetic way. This may affect the decision to regulate process over product.
International Harmonization
Currently, it appears that the US, EU, and Japan, among other nations, have invested significantly in nanotechnology development. Given the large and growing global trade (Environment and Globalization: The Five Propositions) in raw materials, intermediate, and finished goods (Energy and Society: Chapter 9: Capitalism in Theory and in Fact), it is more than likely that products produced using nanotechnologies and products incorporating nanotechnologies will enter into international trade. Furthermore, it is likely that the development of some nanotechnologies will benefit from harmonization and/or interoperability of standards across national boundaries. While several countries are very active in developing nanotechnology standards and regulations development, little discussion has taken place regarding their global harmonization.
Internal harmonization involves four interrelated themes, including: (1) the kinds of standards and regulations that will need to be coordinated globally; (2) whether, and if so, how best to ensure that the interests of countries other than those identified above are included in global standards harmonization; (3) whether certain standards can remain local/national in scope; and (4) preferred ways of moving the process of international harmonization forward.
From National to International
Before discussing what kinds of voluntary standards and government regulations will need to be harmonized globally (International Climate Treaties), we should deal with the concern about standards proliferation, especially when many countries lack adequate enforcement capacity. It is dangerous to focus on standards-setting while neglecting enforcement capacity, which is equally important. At the same time, inadequate enforcementshould not limit the drive toward for harmonization, but should be coterminous with building capacity.
For international harmonization to be possible, it is necessary to first deal with national standards and regulatory frameworks. Each country still has obligations to its own population. Harmonization will be difficult because it must be compatible with many diverse cultures. How to deal with these cultural differences in constructing global standards is unclear. Furthermore, the identification of priority areas will be difficult since public concerns will differ from country to country.
Participation in the forum that ISO provides is critical, particularly for the US, which is large enough that failure to participate could injure its reputation as a global trading partner; moreover failure to participate, or indeed provide access to participation, raised serious concerns among some workshop participants regarding potential social justice issues pertaining to the distributive effects of various standards alternatives under consideration by parties to the ISO standards negotiations. In the agriculture and food sector, Codex and/or the International Plant Protection Convention are more relevant. ISO has experience in developing product, nomenclature, process, and test standards. Technical standards will be needed to determine whether particles are found in a product and can be released in a consequential manner. Within the US, participation in ANSI deliberations should be encouraged.
Some participants noted that the ISO process that allows one vote per country can be very political. Thus, it is not always possible to develop global standards using this format. Alternative approaches to international standards-setting should be considered. For example, the American Society of Mechanical Engineers (ASME) sometimes functions as a default international standards-setting body, but is not always recognized as such.
Harmonization will also require communication among different bodies. This will raise the issue of data privacy and transparency (Global citizens movement), especially since some nanotechnologies (e.g., Radio Frequency Identification [RFID]) will enhance the ability to transfer data. Harmonization of information gathering for processes and products, in order to establish consistent and reliable ways to collect information, will be needed.
Furthermore, if there is too much pressure to keep the cost of standards low, then there is insufficient cost recovery to allow for the constant development of new and evolving standards. Standards are often copyrighted or otherwise protected by standards owners (e.g., ASME standards). This can be a barrier to adoption. There are cost barriers associated with ISO, ASME, ASTM, and other standards.
Which Standards?
In harmonizing standards at a global level, there is considerable agreement that the main focus needs to be on public health (Climate Change and Public Health) impacts. Labeling, quality issues, and environmental issues also need to be harmonized internationally. Worker safety is a more complex issue as standards-setting works differently in different places and organizations. However, standards need to be harmonized where they concern risk, exposure, and waste disposal. Product standards do not necessarily need to be harmonized but perhaps process standards do.
If nanotechnology standards evolve from current standards, then there will be a combination of national regulations informed by international standards. It might be possible to begin by agreeing on principles for standards rather than on the specifics. International standards have more potential to become politicized while national standards can be developed in a manner that is relevant to local conditions. Worker health and safety standards are complex. For example, high worker standards can be an economic (Mainstream economics and the political process)disincentive. This might be a reason to keep this local or national in scope. In nanotechnology applications, if there are environmental consequences, they must be related to local and national situations. However, nanotechnologies exhibit unique features and do not have national boundaries. Some nanoproducts, if persistent, could have international implications if they are released into the atmosphere (Atmosphere). Therefore, the question of the right of a country to refuse to be in contact with the product needs to be addressed. There also is the issue of the right of a government (Government-owned enterprises)to refuse exposure of its citizens to certain materials.
Which Countries?
In multilateral standards-settings processes, the exclusion of some countries for practical reasons is a limit to international harmonization. Also, the cost of the standards can make them unavailable to developing countries and prevent them from participating at the international level. Consequently, cost recovery is a really important issue.
In any case, developing countries should have a say in international nanotechnology standards development, even if they lack capacity to enforce the standards. This is important since how standards are set often controls whose exporters can enter a given market. One means to address this might be for public agencies in industrial nations to set aside research or intellectual property for developing countries as a tax (article).
Regional discussion also might help strengthen the position of developing countries at the international level. The most important thing is to establish localand regional standards and then to navigate international barriers. Thus, better national planning is a central issue. Physical presence does not necessarily produce participation; there also has to be preparation beforehand (e.g., in determining what questions should be asked). Finally, given current concerns over national security, the inclusion of all nations may be contentious.
Integration of Operational Standards
Nanotechnologies pose new challenges for operational standards and regulations. Additionally, regulations must ensure that the health and safety of workers and consumers are protected and that [[environmental protection]s] are developed and enforced as needed. In the past, these issues were the subject of separate regulations and regulatory agencies (e.g., in the US, OSHA (National Institute for Occupational Safety and Health (NIOSH), United States), EPA, FDA, and USDA (Department of Agriculture (USDA)) each hold responsibility for different aspects of the regulation of agriculture (Agriculture and Climate Change) and food (Food Security) products). Integration of diverse standards regarding nanotechnologies is likely to pose new challenges for governmental regulation and non-governmental standards.
Three interrelated themes around integration emerge, including whether: (1) both private standards-setting and governmental regulatory agencies that have not historically worked in cooperation will begin to do so to effectively integrate the regulation of nanotechnology; (2) procedures can/should be established to ensure adequate integration among different agencies in the emerging areas of nanotechnology; and (3) unique challenges will be raised by this issue, and if so, how to identify the best strategies for addressing them.
Integration among Agencies
Agencies may start to work together as the need arises, but this is not likely to happen on its own. Instead, mechanisms must be established for interagency cooperation where it has not occurred before. Success in this endeavor is key, so we need to look for examples where different agencies have worked together cooperatively on regulations and then identify earlier successes and failures.
Currently, in the US there is very little or no interagency talk on emerging nanotechnology standards and regulatory needs. For example, the EPA recognizes that there will be waste (Modern environmental health hazards)to regulate, but it does not know much more than that. The EPA does not know what the waste will be or how much of it there will be. There needs to be integration among agencies to better enable them to understand this, and to be most effective, such integration would need to involve more than just regulatory agencies alone. The National Nanotechnology Initiative (NNI) -- a multi-agency U.S. Government program that coordinates Federal efforts in nanotechnology – could potentially provide this service, if not serve as a model for such efforts.
Another good example is nano-sensors and RFID tags, where there may be a need for the United States Department of Agriculture to integrate its standards with those of the Federal Communications Commission. This probably has never happened before. Still other agencies probably are not thinking about nanotechnology at all. For example, it is unlikely that Grain Inspection, Packers, and Stockyards Administration has considered the importance of nanotechnology regulation. Likely, it will need to do so. Therefore, mechanisms will need to be established to help them begin to see their role in this regulation.
Compounding these problems, there is a history of lack of communication among regulatory offices. Better communication methods will need to be fostered to overcome this history. A good place to start might be with integrated computer systems. A big challenge is information sharing, so the easier and more efficient information sharing between the agencies can be made, the better. Designing a Central database appears to be a useful first step. There is tension between some agencies when it comes to information sharing (e.g., who gets the credit?, etc.). There needs to be a focus in overcoming this and achieving information sharing.
Often, barriers between agencies occur at specific points. If communication can be facilitated at those points, the entire interagency process can be enhanced. Furthermore, each individual agency may have insufficient funds to accomplish its mandated work, let alone to set up cooperative networks. Additional funding will be extremely important to success. Ultimately, two things are needed: someone with the authority to integrate and an agency to catalyze the process. For these reasons, some argue that in the US some entity other than the NNI should play this role, although its NNCO does have a similar function within the NNI. However, creating a completely new agency could conceivably generate more confusion. The bigger question remains which agencywould take the lead and act under what mandate?
There is a conflict between top-down and bottom:up approaches to integration. However, it is difficult to provide an example of a top down approach that has worked well. The Department of Homeland Security has a top-down model with enormous power and vast resources, yet its success is questionable. Therefore, bottom:up models of integration are needed. Moreover, the Coordinated Framework for Biotechnology (Office of Science and Technology Policy 1986) should be examined to determine if a similar approach would work with a larger number of agencies.
Integration with Private Sector
Five of the top ten food companies are major nanotechnology investors. These and other private/corporate investors have a great deal of information that is likely to be useful to the standards-setting process. Integration among agencies and also among all supply chain actors is also important. Wal-Mart is now a de facto standards-setting body for quality standards.
Standards and regulations have historically ignored the complexity of the supply chain. Yet, integration with private sector players could help establish new approaches similar to Hazard Analysis and Critical Control Points, commonly used in the food industry, along the supply chain. Instead of waiting until a product is complete, one can test it along the way.
At the same time, it is important to be wary of the role that people/companies with a financial stake in nanotechnology play in the integration of operational standards with worker and consumer health and safety (Research, Public Health and Biologically Relevant Exposures), and environment regulations, especially in sectors where industry giants may hold significant power over standards-setting.
Various US government agencies are collaborating with ANSI and ISO in standards development. ASTM’s E-56 committee is also involved in nanotechnology standards development, and has recently released its standard ‘E-2456, Terminology for Nanotechnology’ (ASTM E-56 2006). There is considerable overlap in membership among these and other standards organizations, and they may serve as a fruitful place to begin the discussion of standards integration.
Finally, global integration will require cooperation among competing institutions. Typically, the tension that results from competition limits cooperation on regulation. Additionally, who integrates with whom becomes a point of contention.
Challenges: Old and New
The greatest challenges are not so much unique, as they are persistent yet unsolved. Such is often the case with new technologies (Technological Nightmares (Lecture): Nanotechnology). If we can solve the problems presented here, we will have taken a large step towards solving the regulatory challenges associated with all emerging technologies.
These persistent unsolved challenges lie largely in the complex social dimensions of technology (Technological Nightmares (Lecture): Nanotechnology). The proposed National animal identification system is a good example. Regulatory agencies thought this would be a simple matter of organizing a database and implanting tracking devices. However, it quickly became a larger social issue involving government knowledge of herd size, location, transport, etc. Also, with such a database a Disease outbreak beyond a farmer’s control can be traced back to the farmer. This poses potential issues of liability and social stigma.
Yet another challenge to nanotechnology is reviewing products already on the market for safety and efficacy by regulators. Someone needs to set standards for the nanoparticles in certain tennis rackets, but it is not obvious who should do this. With nanotechnologies it will be important to set standards in parallel with product development so as to avoid this in the future. Also, nanotechnology waste that has not been evaluated for health, safety and environmental risks already exists. Companies are unlikely to use their R&D funds for this type of research; a governmental body needs to do it, and sooner rather than later.
In addition, the hazardsassociated with nanotechnology are largely unknown. This makes it difficult to assess how to proceed. Standards are needed for working in situations of uncertainty. Some form of the precautionary principle might be appropriate, at least until the hazards are more well-defined. Another challenge is the difference in money available to fund product R&D and money (Valuing environmental costs and benefits)available to fund worker, consumer, and environmental risk research.
A final challenge is commercialization of university research projects (spin-offs, private research parks, etc.). Nanotechnology research projects often involve an intensification of this trend. Historically, university research has been regulated differently than commercial research. However, as the boundary between universities and commercial firms blurs, the regulation of university research needs to be rethought. This provides yet another reason for integrating operational standards with consumer, health and safety regulations.
Participation and Transparency in Standards-Setting
Historically, both private standards-setting and governmental regulatory bodies have worked with scientific experts and business. This approach has generated robust national and international standards regimes that have simultaneously advanced and protected proprietary interests while facilitating global commerce and trade. However, this approach is coming under increasing public scrutiny. First, the level and nature of risk that consumers and workers find acceptable may be different from that which business and experts consider appropriate. Second, the non-public nature of some standards or regulatory development can create an impression of collusion and secrecy between industry, experts, and government that can undermine public confidence in standards and standards-setting bodies.
Three interrelated themes emerge in the topic of participation and transparency in standards-setting, including whether and how: (1) public participation should be increased in nanotechnology standards-setting processes; (2) limits should be placed on such participation; and (3) standards-setting and regulatory processes can be made more transparent.
Defining and Operationalizing the Concept of Standards
To increase public participation in standards development and implementation, the concept of standards will first have to be defined and operationalized so that the participants are responding to the same basic idea. Key dimensions for clarification include:
- Standards vs. Standardization. The terms ‘standards’ and ‘standardization’ refer to distinct concepts. For example, ‘standards’ may be used either to standardize or to differentiate among products, processes, and procedures. Participants in standards-setting processes must be made aware of this distinction so they clearly understand the intended purposes, outcomes, and potential consequences of their participation.
- Formal vs. Informal Dimension. One dimension that must be clarified immediately is the degree of formality of the standards in question. For example, where along a continuum extending from legally binding restriction and technical proscription at the formal end to social convention at the informal end are such discussions to occur? Traditionally, the more formal standards dimensions provide less room for full public participation; conversely, social conventions are by nature negotiated through open and transparent public interaction.
- Public vs. Private Dimension. The same may be said of public versus private standards, where private standards are typically negotiated by less public or participatory means.
- Technical vs. Strategic Dimension. The very concept of standards needs to be presented publicly as a socially negotiated and strategic phenomenon, rather than solely as the specification of technical attributes or criteria. Standards first need to be recognized as strategic devices that are negotiated among and reflect the interests of participating groups. Standards are thus simultaneously technical and social phenomena that both reflect and are responsive to the broader participation of potentially affected groups.
Define and Identify Potentially Affected Groups
The bigger questions involve how to identify who the potentially affected groups are, the preferred participatory processes once they have been identified, and clarifying the goals of the process. Some of the key questions that will have to be addressed up front in each of these areas include:
- Identification. Are these demographic categories of people? Are they defined geographically, socially, culturally, by spheres of economic activity? Do they ‘self-identify,’ or are they identified by others? And how do companies, private industries, etc., fit into this mix?
- Process. Once potentially affected populations have been defined and identified, is their participation a function of attending formal standards-setting events, or is it incumbent upon standards-setting organizationsto engage in outreach to obtain information from these groups, however defined? And in any of these cases, how does one know if one has been successful? The process of participation must be set to meet the expectations of those who are to participate in that process; otherwise potential participants may be less likely to want to participate and doing so will not meet their expectations.
- Goals. What are the standards for participation in standards-setting processes? What principles guide the process? What is the goal? ‘Better’ decisions? Broader representation in decision-making, regardless of the quality of those decisions? Equitable distribution of impacts, costs, benefits? Greatest good for the greatest number of people? Economic efficiency? These things will need to be agreed upon, or if not agreed upon, then they will need to form the basis for public discourse concerning the principles of participation prior to the implementation of participatory strategies in standards-setting processes.
There are plentiful reasons to pursue greater public participation in standards-setting and regulatory processes, including for example, that (a) it’s the right thing to do, (b) it fosters public trustin, (c) it can lead to greater public protection from unforeseen risks (Guide to Risk Assessments and Public Health Assessments) given less participatory processes, and (d) it provides for greater public insight into regulation (Role of science in regulation).
The public may have questions that the regulators have not considered. Regulation is a long-term affair while public engagement is too often seen as something to be tacked on at the end of the process. At the same time we need to recognize that this can slow the regulatory process. One might want to engage in different forms of participation for different reasons. There is a need to be sensitive to culturally appropriate forms of participation. For example, experience conducting public participation among Amish and Native American communities, where collective decisions are often framed through the counsel of respected elders, suggests that ‘one-person, one vote’ models are not universally accepted, nor are random samples or statistical representation necessarily desirable (Stone 2001).
- Incentives. From a company perspective there has to be a marketplace aspect. There needs to be a clear benefit for encouraging broad participation in whatever process is selected. If this cannot be established, then there is little incentive to do it. Then it comes down to being required, or forced to do so, and this is not always desirable in the absence of clear benefit. An economic basis for participation must be established from a company perspective. Is it going to be better than what the market would provide? How is this made clear?
Preferred Models of Participation
Many models exist for public participation and may be adapted in one way or another to meet the needs of participation in standards-setting processes as well as the expectations of the participants. The International Association for Public Participation is a good repository of such information.[4]
Models may range from highly centralized events, such as public hearings, to highly decentralized processes, such as community extension services. These may be highly facilitated/mediated or analytical/deliberative events. In mediated processes, participants resolve a dispute on their own without any 'decision' being made by a chairperson or a judge. The resolution in mediation may incorporate agreements on legally irrelevant and often emotionally charged issues. Deliberative processes, on the other hand, are more legalistic or legislative in nature, and typically avoid legally irrelevant and emotionally charged issues. Some examples of successful models used in other contexts include the following:
- Nano Jury’s “Mutualistic Engagement” model, builds upon the UK (UK Department for Environment, Food and Rural Affairs)experience with its “GM Nation” effort and is presently being applied to public engagement around emerging nanotechnologies;[5]
- The South Carolina Citizens’ School of Nanotechnology is a model of engagement that is particularly well-adapted to public education on nanotechnology;[6]
- The USDA (Department of Agriculture (USDA)) Cooperative State Research, Education, and Extension Service (CSREES) provides information concerning decentralized extension-based approaches to community outreach and education that are broadly applicable to public dialogues concerning nanotechnology standards;[7]
- The Risk Perception Mapping (RPM) model, developed primarily for social assessment in nuclear waste facility siting, is a decentralized and ethnographic approach that may work well in assessing the potential social impacts associated with siting nanotechnology manufacturing facilities. For example, it can address issues such as how such facilities will be perceived and acted upon by potentially affected groups. Will such facilities be perceived as analogous to other historically unwanted land-uses, which in turn are used to similarly frame social response to the proposed nano facilities? Or might they be perceived as analogous to other more positively framed facilities and events? Moreover, RPM has been used to help identify and correlate the spatial and cultural (geo-cultural) characteristics of potentially affected populations with their project-specific risk perceptions and policy expectations.
Less clear is how such models would work within standards-setting processes. One thing CODEX has done for international Non-Governmental Organizations is to develop standards through electronic workshops rather than physical working groups. Perhaps a hybrid model mixing and matching various elements of these could be developed for specific standards-setting processes.
Public Meetings are Insufficient
Public meetings provide a venue where people can publicly express themselves, make impassioned pleas on behalf of their communities (Communicating climate change motivating citizen action), demonstrate their commitment and dedication to and concern for their community’s well-being, but they do not necessarily provide information regarding the distribution of concerns among a population. One cannot assume a speaker speaks for a community of interest, and the claims made at such meetings should not necessarily be considered public participation.
Drivers of Information/Insight Gained through Public Participation
What are the drivers of the information being sought through public participation, and what insights are to be gained through such processes?
- Risk Perception. The social impacts literature suggests that such impacts occur to the extent that people perceive themselves to be at risk (Risk society) from something. Risk perception is an important driver in standards participation. For example, one might ask what risks and impacts do potentially affected groups associate with the phenomenon around which standards are being developed, and perhaps more importantly, what are the modes of risk impact.
- Risk perception analogues. The public perceives risks of new technologies through experience with applications of earlier technologies. Illustrative examples are failures such as Chernobyl, Exxon Valdez, and Bhopal, where the concern was not with the technology (Response and clean-up technology research and development and the BP Deepwater Horizon oil spill) per se, but rather with management (A competent and nimble regulator: a new approach to risk assessment and management)of the technology. This introduces a new dimension to ‘risk identification’ and management, extending it beyond purely technical considerations and into the realm of social experience with analogous technologies and projects. It also introduces issues surrounding public trust in the institutions charged with managing the risks associated with the technology generally, or with a specific project or application of that technology.
- Trust. Bernard Barber’s (1983) work on trust is instructive here. Barber links concepts of trust with public expectations about the future, specifically, (a) ‘the persistence and fulfillment of the natural and moral social orders,’ of (b) ‘technically competent role performance,’ and that (c) ‘partners in interaction will carry out their duties in certain situations to place others’ interests before their own,’ what he calls ‘fiduciary responsibility.’ Public participation is often marked by a disjuncture among these expectations, particularly (b) and (c), where on the one hand scientists and technical ‘experts’ typically frame their risk discussions around assumptions and demonstrations of technical competency – e.g. ‘trust us because we are technically competent,’ and on the other hand, potentially affected publics typically frame the issue in terms that include but extend beyond technical competency to encompass the ‘fiduciary responsibility’ they see as inherent to risk management – e.g., ‘can this institution be trusted to place broader public interests above its own immediate concerns?’ As such, Barber’s work is instructive concerning potential disjunctures in public participation in standards making, where there is likely to be a tension between the purely technical dimensions of the standard and the broader fiduciary concerns. How might these be integrated to build public trust in not only the standard itself, but the process through which it was developed, and indeed perhaps the technology itself?
In this sense, social experience with nuclear power, biotechnology (Biotechnology in Animal Agriculture: Status and Current Issues), wireless communications, etc., would be helpful for understanding the kinds of risk perception analogues that will likely drive public participation around nanotechnology, including the development and implementation of standards in this area. This may not generate ‘better’ standards per se as much as a range of ‘different’ standards, niche standards that respond to the values and expectations of distinct communities-of-interest.
Risk Communication
Although public education (Environmental Contaminants and Health: Communicating the Science to the Public (Course)) is important to an informed public dialogue on nanotechnology, the participatory issue should not exclusively be about technical understanding of the phenomenon around which specific standards are being developed but equally about social understanding of how a person and her or his social network stands to be affected by both the phenomenon and the standards being developed around it. It has to work both ways. Decision-makers (standards-setters) must remain open to being educated by participants about the social contexts of their concerns — contexts that ultimately will have to be addressed in the standards that are promulgated.
Risk communication is a two-way street and must occur early in the process to help frame social contexts of risk perception. This, in turn, will help clarify for decision-makers the preferred subjects of risk assessments and socially appropriate risk management options. Standards-setting processes have to be collaborative, and participation is a vehicle for that. Otherwise, one has the old linear model of experts deciding what counts and the public reacting to their decisions. The key will be translating the processes used into socially responsive policies and standards – if that is the goal. The International Risk Governance Council is a good example of an organization that deals with such issues, and it has recently released a white paper on nanotechnology risk governance that is informative for public participation in nanotechnology standards development[8].
Limits of/to Participation
There should be procedural but not substantive limits to participation. Some of the key issues to be considered include:
- Proprietary Interests of Participants. Proprietary interests of participants must somehow be protected. Multiple layers of proprietary interests are likely to influence the process. Moreover, transparency (Sustainable Society Index)itself may actually be seen as a disincentive to participate, particularly if transparency means that trade secrets or other proprietary information could be publicly revealed. This is a timing issue: done too early in the process could have the opposite of the desired effect, where industry particularly is unwilling to participate. Then the process is likely to be seen as dishonest rather than transparent. This is a paradox that is difficult to overcome.
- Human Subjects. There is a need for full disclosure of purpose and use of information obtained through participation. In addition, there must be assurances that data will be used in only certain ways. Some key questions that must be addressed are: How can this be ensured? Who is responsible for situations where such disclosures are violated? How are disputes arising from this to be adjudicated?
- Saturation, Co-optation, and Accountability. There is a need for streamlining among all the many stakeholder organizations and potentially affected groups. Participationcan be costly and time consuming. In the US it tends to be industry representatives who have the financial support to attend these events. If there are thirty meetings a year, which are the ones most worth attending, in terms of both affordability and ability to influence outcomes? Answering this question presumes that one can anticipate outcomes. Yet, one cannot know whether a specific meeting is the one in which it is most worth participating. The participation ‘market (Limitations of markets)’ can quickly become saturated, with no clear direction of to whom to turn or toward what ends the process will lead. For example, the interests being served will likely be reflective of the interests of those who coordinate and implement the event. That is, those who control participation can shape it in their image. The perception that one group’s interests can be co-opted by another’s is a potential disincentive to participation. It creates a cynical perception of the process and ultimately of the decisions reached. Yet, someone has to make these decisions, and not every interest will be equally served. This raises related issues regarding those interests consistently underserved in standards-setting processes.
- Scale – Local to International. Scale presents another potential limit to participation – not everyone can or perhaps even should participate. Can local input be scaled up to national and international dialogues, and vice versa? This could become quite daunting and the complexity itself could serve as a disincentive to scaling up participation.
- Equity and Social Justice. At some point decisions must be made. One has to have milestones for progress in decision-making, and yet at the same time there are many publics that are difficult to reach. Equity issues may be sacrificed for the sake of expediency. Simple ‘majority rules’ solutions may not adequately address equity, particularly in instances where the ‘minority’ views are consistently discounted. There is a need to protect minority perspectives from a ‘tyranny of the masses,’ a social justice dimension that should not be overlooked, but often is. In this sense, current limits on participation may actually have to be expanded in order to allow for greater discussion of equity and social justice. But determining who will make that call, and on what grounds, is the subject of broad public dialogue in and of itself. Key questions in this area include (a) how equity issues can be addressed procedurally, and (b) whether participants are willing to accept outcomes or decisions that they might consider inappropriate. This condition will have to be understood by all parties going into the process. Otherwise the process will unravel and simply become the domain of a self-selected subset of interests that control the process. This cannot legitimately be called ‘participation.’
Toward Greater Transparency in Standards-setting Processes
In standards-setting and regulatory processes, no open-source mechanisms exist in which the public can clearly see the process and is welcome to be part of it. This would assume an educated public, at least to some degree, so a truly open source mechanism could only be implemented upon the shoulders of a broader public education mechanism. However, as noted previously, public education is a two-way communications issue in which the point is not just to educate the public on the technical aspects of standards issues, but to be educated by them concerning the social contexts of concerns that ultimately will have to be addressed in the standards that are promulgated.
- Formative Evaluation. Formative evaluation can help to increase transparencyin standards-setting processes, provided the parties involved agree that increased transparency is desirable. Key evaluation questions concern: (a) establishing a clear definition or understanding of ‘transparency,’ specifically its goals and procedural objectives, and (b) how one knows when these have been met. The answers to these questions will likely vary by stakeholder interest, so this dialogue needs to occur independently of the standards-setting process. Answers to these questions will help establish appropriate models of participation that can then be pursued to maximize transparency as so defined. This should be an iterative or formative process in which outcomes inform the implementation of future processes, that is, in terms of the definitions, goals, and procedural objectives of both transparency and the participatory procedures used to obtain it.
- Educating the Public on Nanotechnology. The South Carolina Citizens’ School of Nanotechnology provides a good example of educating the public about nanotechnology generally, but turnout has been quite low to date. Also, it is unclear how representative of broader publics such techniques actually are, or whether they have even been conceived to address representational issues. Clear representation in educational programs is important – determining who speaks for whom, and how (or whether) each voice in the process can be weighted, and to what effect. The South Carolina program team is presently revising the Citizens’ School process to explicitly address these issues.
- Educating Decision-makers on Social Context. National representatives to CODEX and other groups have a responsibility to collect information from their respective publics. But there are no consistent procedures whereby national organizations are expected to interact with their publics. The information is inconsistent, and there is little guidance regarding how that information will actually be used to help shape standards decisions. A recent report of a joint workshop of the Food and Agriculture Organization (FAO) and the World Health Organization (WHO) indicated that in such cases there must be room for minority opinions and decision-makers must be accountable for why such opinions may not be factored into the decisions reached (FAO/WHO 2004).
In the UK, DEFRA (Department of Environment, Food, and Rural Affairs) has utilized focus group models to increase public accessibility to nanotechnology discussions, and this could be adapted to increase transparency of nanotechnology standards deliberations, or at least public understanding of how these processes work[9]. A public advisory board could work as intermediary between the formal standards-setting process and the multitude of voices that could potentially demand a formal role in the process. The interaction between the advisory board and the public would be very transparent. The board would then take that information to the standards committee. Someone still has to make decisions, though, and the public is left largely to react to rather than collaborate on the decisions reached.
Conclusions
Timing
Given the concerns noted above, standard-setting and regulatory activities should start early and be seen as a strategic and iterative process. Indeed, all regulation and risk assessment (Guide to Risk Assessments and Public Health Assessments) has operated on the basis of incomplete knowledge. Regulators should note that they are doing their best within the current framework, rather than employing a ‘trust us’ approach. This will involve an admission by regulators of how much we do not know. At the same time, it will require a significant increase in funding to identify risks and to engage in formal risk assessments. The NNI reports that four percent (roughly $40 million) of its FY 2006 budget was dedicated to R&D aimed primarily at understanding and addressing the potential risks posed by nanotechnology to health (Ecosystems and Human Well-being: Health Synthesis: Summary for Decision-Makers) and the environment (Global Environment Outlook (GEO-4): Reader's Guide) (NNCO 2005; PCAST 2005).[10] A recent report of the Woodrow Wilson Center’s Project on Emerging Nanotechnologies, however, contests the NNI’s $40 million figure, suggesting only $11 million is actually geared to ‘risk relevant’ research. Still, semantic issues aside, Wilson Center researchers claim neither amount is adequate and instead recommend a minimum funding increase of $50 million over each of the next two years (Maynard 2006a, 2006b). If risk assessment moves slowly while product development moves rapidly, we may be more likely to experience a calamitous incident.
Initially, standards and regulations should focus on laboratory research and research institutions, as well as on reporting of incidents. Simultaneously, funding for risk research, currently inadequate, should be increased. Since rulemaking is a lengthy process, specific deadlines should be established for preliminary standards. It should begin with conservative and inclusive standards, so as to reduce exposure to potential hazards, but should not be so conservative as to bring research to a halt. Time is of the essence. The later standards emerge, the more vulnerable everyone involved will become. Timing is also related to transparency (Social and environmental responsibility of corporations)in that a rapid decision about the need for process or product standards may influence transparency and disclosure requirements. This will in turn affect public opinion about nanotechnology and the level of public control over decisions about nanotechnology.
Product vs. Process
Standard-setting for nanotechnologies is going on in many nations. While the creation of globally acceptable international standards for nanotechnology products, processes, research, environment, and health and safety is still far off, cooperation and information exchange need not wait until individual national standards and regulations are established. Nor should national standards and regulations be avoided because one is worried about competition, or because international standards are insufficiently developed. Indeed, for global trade (Environment and Globalization: The Five Propositions) at the very least everyone must use the same language. Such global collaboration can include identification of risk factors, means for public engagement, Public opinion polling, and public education (Environmental Contaminants and Health: Communicating the Science to the Public (Course)). In addition, trade involving certain Nanoproducts (e.g., Radio Frequency Identification Devices) will need to take into account and develop appropriate methods for preserving privacy with respect to the vast amounts of data generated in the process, as well as various obligations under the World Trade Organization.
Product/process standards (Ozone nation: EPA standard panned by the people)may also be affected by the initial ordering of the standards, i.e., the sequence in which product and process standards are introduced. There is still room to debate whether nanotechnology processes should be subject to standards first or whether product standards should be developed first.
Necessarily, the goals for and safety of nanotechnologies will affect the standards developed for them. In this way, the standards will depend on the nanotechnology in question. There is disagreement as to how cautious to be. Some argue that technologies should be treated as potentially harmful until demonstrated to be safe, while other argue that we must experiment both with the technologies and with the standards. In the end, the practical products and processes that are in use or about to be used will force us to establish standards.
Process and product standards should vary by sector only if what works within that sector differs from other sectors. In other words, if there are similar goals across sectors, then there should be consistency. Imposition of a one size fits all approach is unlikely to succeed. The ability to experiment should be preserved.
International Harmonization
Every debate over harmonization is about national differences in standards and regulatory frameworks. At the same time, public demand for transparency in the harmonization of standards is likely to be a driving force. It might also be useful to talk about what values we wish to promote. Coordination with the United Nations Development Programme’s Millennium Development Goals (United Nations Millennium Declaration),[11] for example, might be worth pursuing.
Integration of Operational Standards
One must ask whether standards for research and development should be integrated with consumer, worker and environmental regulations or not. Such a move may slow research and development, but with the unique concerns associated with nanotechnology there is reason to consider this option. If nations or international bodies move in this direction, it will be necessary to identify the forces that prevent effective integration and work to overcome them. Furthermore, overcoming existing power, hierarchy and domination in the standards/regulation development process is important to attain publicly acceptable results. One way to address this is by developing ways for NGOs and other citizen groups with limited resources and diverse objectives to play a meaningful role.
Participation/Transparency
Public education and engagement should proceed concurrently with the standards-setting process. Currently, public awareness of nanotechnologies is limited although growing. More public education is needed if a true dialogue is to take place, although this too is an iterative process. The more that dialogues take place, the more awareness will increase. In 2003 Congress mandated education on this issue (USC PL 108-153, 2003), but little has happened to date. However, successful models of rapid public education do exist, including that of the Cooperative Extension Service. These should be used as needed. Public interest organizations should be constructively involved in the process from its inception, although limits of time and resources make it impossible to include ‘all interested parties.’ Encouraging high levels of participation will likely reduce both negative and adversarial aspects of the process. Potentially affected parties need to be identified rapidly, so that means for mitigation can be identified more easily.
Acknowledgments
Event planning and report preparation are truly collaborative efforts and this workshop was no exception. Special thanks are due the following people for their roles in helping to plan, organize, and host the workshop, and for their efforts in preparing this report of workshop proceedings: Julie Eckinger, Mary Keyes, Nicole Schoendorf, and Jill Crandell for administrative, logistical and secretarial support; graduate research assistants Sho Lisa Ngai, Norbismi Nordin, Brian Depew and William Hannah for their help in preparing this report based on their notes and recordings made at the workshop; Cowles House Manager Peter Lechler and Presidential Events Coordinator Theresa Pharms; Agrifood Nano Project team members Les Bourquin, Lawrence Busch, Kenneth David, Brady Deaton, John Lloyd, Susan Selke, John Stone, Deepa Thiagarajan, and Paul Thompson; the Department of Sociology, the Department of Community, Agriculture, Recreation and Resource Studies, the College of Social Science and the Michigan Agricultural Experiment Station — all at Michigan State University; and, of course, the workshop attendees (listed on the last page of this report) without whose enthusiastic participation the workshop would not have been possible.
Immediately following the workshop the MSU Agrifood Project team assembled a presentation and poster of workshop goals, methods, and preliminary outcomes, which they delivered at the nanotechnology-related meetings and conferences of various stakeholder groups, including the U.S. Food and Drug Administration, the American Society for Testing and Materials, the National Institute for Occupational Safety and Health, the National Council for Science and the Environment, and the Society for Applied Anthropology. The following attendees of those meetings, together with these other interested parties, offered voluntary review of the penultimate draft report: Katherine Albrecht, Jeanne Bank, Knut Blind, David Brenner, Richard Canady, Daniel E. Collins, Vicki Colvin, Abbey Dilley, David Forman, Bernard Gadagbui, Sandra Geibel, Charles Geraci, Gina Gerritzen, Patti Glaza, Bob Gruenig, Peter Hatto, Mark Hoover, William Kajola, Matthew Kearnes, Adam Keithley, Sandy Lawrie, Phillip Macnaghten, Zahra Meghani, Celia Merzbacher, Sonia Miller, Madeline Narwar, Elizabeth Nielsen, Gregory Nilsson, Dianne Poster, Maria Powell, Allan Rawle, Nick Reo, Rish Riswadkar, Allen Robison, Mihail Roco, Robert Scace, Rolf Schneider, Hideo Shindo, Carol Steele, Marvin Stone, Clayton Teague, Gregor Wolbring.
Finally, we would like to thank the National Science Foundation. The workshop and this report were supported by a National Science Foundation/Nanoscale Interdisciplinary Research Teams Grant: Building Capacity for Social and Ethical Research and Education in Agrifood Nanotechnology (SES-0403847). The views expressed here are those of the workshop participants and reviewers, and do not necessarily reflect those of the National Science Foundation, Michigan State University, or the participants’ employers.
Notes
1. The Coordinated Framework for Biotechnology was established by the US government in 1986 to regulate biotechnology products.
2. In the US context, the term ‘standards’ is often applied to both voluntary standards set by various private or and non-profit organizations as well as to mandatory public regulations set by government agencies. In contrast, in the EU voluntary standards are usually contrasted with government regulations. However, in recent years, in part as a result of increased global trade, the distinction between standards and regulations has become blurred. Many nominally voluntary standards have become de facto mandatory. In this document we follow the US usage, and the usage employed during the workshop, distinguishing where necessary between voluntary and mandatory requirements.
3. David, Kenneth and Paul B. Thompson eds. (Forthcoming late summer 2007). What Can Nano Learn From Bio?: Social and Ethical Lessons for NanoScience from the Debate over Agrifood Biotechnology and GMOs. A volume of the Food Science and Technology
series. Elsevier.
4. See, e.g., http://www.iap2.org/index.cfm
5. See, e.g., http://www.nanojury.org/intro_mutual.htm
6. See, e.g., http://nsts.nano.sc.edu/outreach.html
7. See, e.g., http://www.csrees.usda.gov/qlinks/extension.html
8. See, e.g., http://www.irgc.org/irgc/_b/contentFiles/IRGC_white_paper_2_PDF_final_version.pdf
9. See, e.g., http://www.defra.gov.uk/
10. According to PCAST, this amount does not include research of a different primary focus but that nonetheless extends knowledge of health and environmental effects of nanomaterials.
11. See, e.g., http://www.undp.org/mdg/
Sources for standards and nanotechnology
Following is a list of key links and references identified by participants during the workshop or otherwise cited in this document.
Links
- How EPA statutes may be used for regulation of nanotechnology: http://www.abanet.org/environ/nanotech/
- The UK’s Department of Environment, Food, and Rural Affairs (DEFRA) has utilized focus group models to increase public accessibility to nanotechnology discussions. This could be adapted to increase transparency of nanotechnology standards deliberations: http://www.defra.gov.uk/
- The Brazilian Agricultural Research Corporation (EMBRAPA) seeks feasible solutions for the sustainable development of Brazilian agribusiness through knowledge and technology generation and transfer: Further information (in English) regarding EMBRAPA programs and publications may be found at: http://www.embrapa.br/English/index_html/mostra_documento
- Former EPA administrator J. Clarence Davies explains why he believes nanotechnology-specific legislation is necessary: http://www.eande.tv/transcripts/?date=030106
- In 2001, EPA convened a ‘National Dialogue on Public Involvement in EPA Decisions,’ which addresses participation and transparency issues from an EPA perspective. Documents pertaining to this process may be found at: http://www.networkdemocracy.org/epa-pip/
- Based largely on input received through its ‘National Dialogue on Public Involvement in EPA Decisions,’ the EPA released its Public Involvement Policy in 2003, the details of which may be found at: http://www.epa.gov/publicinvolvement/policy2003/
- Institute for Agriculture and Trade Policy (IATP) comment to FDA on regulated products containing nanotechnology materials: http://www.environmentalobservatory.org/library.cfm?refid=89139
- The International Association for Public Participation (IAP2) is a good repository of information on models of public participation: http://www.iap2.org/index.cfm
- An examination of health and environmental safety issues is on the webpage of the International Risk Governance Council: http://www.irgc.org/irgc/projects/nanotechnology/
- The International Risk Governance Council’s White Paper on Nanotechnology Risk Governance (June, 2006): http://www.irgc.org/IMG/pdf/IRGC_white_paper_2_PDF_final_version-2.pdf
- Nano Jury’s “Mutualistic Engagement” model builds upon the UK experience with its “GM Nation” effort and is presently being applied to public engagement around emerging nanotechnologies (see, e.g., http://www.nanojury.org/intro_mutual.htm)
- The South Carolina Citizens’ School of Nanotechnology is a model of engagement that is particularly well-adapted to public education on nanotechnology:[1]http://www.nano.sc.edu/outreachandeducation/citizensschool.aspx
- CSREES outlines decentralized extension-based approaches to community outreach and education that are broadly applicable to public dialogues concerning nanotechnology standards: http://www.csrees.usda.gov/qlinks/extension.html
- The United Nations Development Programme’s ‘Millennium Development Goals’ provides a working example for international harmonization around global interests of shared concern: http://www.undp.org/mdg/
Resources
- ASTM Committee E562006 E2456-06, “Standard Terminology Relating to Nanotechnology,” West Conshohocken, PA: ASTM International.
- Barber, Bernard1983 The Logic and Limits of Trust. New Brunswick, NJ: Rutgers University Press.
- David, Kenneth and Paul B. Thompson (eds.)2007 (Forthcoming). What Can Nano Learn From Bio?: Social and Ethical Lessons for NanoScience from the Debate over Agrifood Biotechnology and GMOs. A volume of the Food Science and Technology series. Elsevier.
- Food and Agriculture Organization of the United Nations (FAO) and the World Health Organization (WHO)2004 Report: Joint FAO/WHO Workshop on the Provision of Scientific Advice to Codex and Member Countries. 27-29 January 2004, Rome, Italy, and Geneva, Switzerland: FAO/WHO.
- Mattoso, L. H.C., E.S. Medeiros, and L. Martin-Neto2005 A revolução nanotecnológica e o potencial para o agronegócio. (The nanotechnology revolution and the potential application in agribusiness). Revista de Política Agrícola, 14(4): 38-48, out./dez., (in Portuguese).
- Maynard, Andrew D.2006a Nanotechnology: A Research Strategy for Addressing Risk. Washington, D.C.: Woodrow Wilson International Center for Scholars, Project on Emerging Nanotechnologies.
- __2006b Safe Handling of Nanotechnology. Nature 444, 267-269 (Commentary, 16 November 2006).
- National Nanotechnology Coordination Office2005 The National Nanotechnology Initiative: Research and Development Leading to a Revolution in Technology and Industry. Supplement to the President’s 2006 Budget. Washington, D.C.: National Nanotechnology Coordination Office.
- Office of Science and Technology Policy (OSTP)1986 Coordinated Framework for Regulation of Biotechnology; Announcement of Policy and Notice for Public Comment. Federal Register 51(123), 23302-23350.
- President’s Council of Advisors on Science and Technology (PCAST)2005 The National Nanotechnology Initiative at Five Years: Assessment and Recommendations of the National Nanotechnology Advisory Panel. Washington, D.C.: Office of Science and Technology Policy.
- Rattner, Henrique2005 Nanotecnologia e a política de ciência e tecnologia (Nanotechnology and the Science and Technology Policy). Passages de Paris 2:180–188 (in Portuguese).
- Roco, Mihail C.2003 Government Nanotechnology Funding: An International Outlook. Nanoscale Science, Engineering, and Technology Subcommittee. Arlington, VA: National Science Foundation.
- Stoffle, Richard W., John V. Stone, and Steven Heeringa1993 Mapping Risk Perception Shadows: Defining The Locally Affected Population For A Low-Level Radioactive Waste Facility In Michigan. Environmental Professional 15(3):316-333.
- Stoffle, Richard W., Michael W. Traugott, John V. Stone, Paula D. McIntyre, Florence V. Jensen, and Carla C. Davidson1991 Risk Perception Mapping: Using Ethnography To Define The Locally Affected Population For A Low-Level Radioactive Waste Storage Facility In Michigan. American Anthropologist 93(3):611-635.
- Stone, John V.2001 Risk Perception Mapping and the Fermi II Nuclear Power Plant: Toward an Ethnography of Social Access to Public Participation in Great Lakes Environmental Management. The Journal of Environmental Science and Policy (special issue titled “Environmental Knowledge, Rights, and Ethics: Co-managing with Communities”) 4(2001):205-217.
- Stone, John V., and Amy K. Wolfe2006 Introduction to ‘Nanotechnology in Society: Atlas in Wonderland?’ Practicing Anthropology (special issue on ‘Nanotechnology in Society’) 28(2):2-5.
- United States Congress2003 Public Law 108-153: A bill to authorize appropriations for nanoscience, nanoengineering, and nanotechnology research, and for other purposes. Washington, D.C.: USC 12/3/2003.
Workshop Participants
Fritz Allhoff, Western Michigan University, Philosophy
Evangelyn Alocilja, MSU - Biosystems and Agriculture Engineering
Les Bourquin, MSU - Food Science & Human Nutrition
Larry Busch, MSU - IFAS
Flora Chow, US Environmental Protection Agency
Renata Clarke, Food & Agriculture Organization of the United Nations (FAO)
Robert Clarke, MSU - Packaging
Kenneth David, MSU - Anthropology
Odílio Benedito Garrido de Assis, CNPDIA / EMBRAPA Agricultural Instrumentation
Brady Deaton, Jr., University of Guelph, Agricultural Economics
Thomas Deits, Lansing Community College Science Dept.
Brian Depew, MSU - Sociology
Mark Duvall, Dow Chemical Company
William Hannah, MSU - Philosophy
Jaydee Hanson, International Center for Technology Assessment
Barbara Herr Harthorn, Center for Nanotechnology in Society at University of California-Santa Barbara
Steve Holcombe, Pardalis Inc.
John Koehr, American Society of Mechanical Engineers (ASME)
Kristen Kulinowski, Center for Biological and Environmental Nanotechnology, Rice University
Jennifer Kuzma, Center for Science, Technology & Public Policy
John (Jack) Lloyd, MSU - Mechanical Engineering
Michele Mekel, Converging Technologies Bar Association (CTBA)
Doug Meyer, International Union of Food Workers
Kirsty Mills, Center for High Technology Materials at University of New Mexico
Julia Moore, National Council for Science and Environment
Stephen Mooser, Retail, Wholesale and Department Store Union
Ed Munn, University of South Carolina
Mark Nelson, Grocery Manufacturers of America (GMA)
Sho (Lisa) Ngai, MSU - Mechanical Engineering
Norbismi Nordin, MSU - Packaging
Steven Pueppke, MSU - Michigan Agriculture Experiment Station
Susan Selke, MSU - Packaging
John V. Stone, Institute for Food and Agricultural Standards, Michigan State University
Steve Suppan, Institute for Agriculture and Trade Policy (IATP)
Toby Ten Eyck, MSU - National Food Safety & Toxicology Center
Deepa Thiagarajan, MSU - Institute for International Agriculture
Jim Thomas, ETC Group
Paul Thompson, MSU - W.K. Kellogg Chair in Agricultural, Food and Community Ethics
Jameson Wetmore, The Robert M. La Follette School of Public Affairs
Jane Wilson, National Sanitation Foundation (NSF) International
-->