Geology of asbestos
Contents
- 1 Introduction The genesis of asbestos (Geology of asbestos) fibers as mineral deposits required certain conditions with regard to chemical composition, nucleation, and fiber growth; such conditions must have prevailed over a period sufficiently long and perturbation-free to allow a continuous growth of the silicate chains into fibrous structures. Some of the important geological or mineralogical features of the industrially significant asbestos fibers are summarized in Table 2. More emphasis is given to chrysotile in the following section owing to its total dominance in the industry over the years.
- 2 Geology and fiber morphology
- 3 Chrystal Structure of Asbestos Fibers
- 4 Property of Asbestos Fibers
Introduction The genesis of asbestos (Geology of asbestos) fibers as mineral deposits required certain conditions with regard to chemical composition, nucleation, and fiber growth; such conditions must have prevailed over a period sufficiently long and perturbation-free to allow a continuous growth of the silicate chains into fibrous structures. Some of the important geological or mineralogical features of the industrially significant asbestos fibers are summarized in Table 2. More emphasis is given to chrysotile in the following section owing to its total dominance in the industry over the years.
Geology and fiber morphology
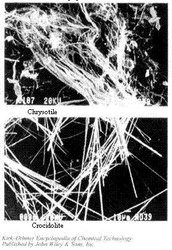
 Figure 1. Unmilled mineral asbestos. (Source: Physical Plant Services, University of Wisconsin-Milwaukee)
Figure 1. Unmilled mineral asbestos. (Source: Physical Plant Services, University of Wisconsin-Milwaukee) Only three varieties of amphibole fibers will be discussed because: crocidolite and amosite were the only amphiboles with significant industrial uses in recent years; tremolite, although having essentially no industrial application, may be found as a contaminant in other fibers or in other industrial minerals (e.g., chrysotile and talc).
Chrysotile
Chrysotile belongs to the serpentine group of minerals, varieties of which are found in ultrabasic rock formations located in many places in the world. Chrysotile accounts for only a small percentage of the minerals found in these rock types. Chrysotile fibers are found as veins in serpentines, in serpentinized ultramafic rocks, and in serpentinized dolomitic marbles. It has been suggested that the ultrabasic rocks, containing olivine, Magnesium-rich pyroxenes, and amphiboles are first altered by hydrothermal processes to form the serpentine minerals; in a later metamorphic event, the serpentines are partially redissolved and crystallized as chrysotile fibers. Clearly, the genesis of each chrysotile deposit must have involved specific features related to the composition of the precursor minerals, the stress and deformations in the host matrix, the water content, the temperature cycles, etc. Nonetheless, it is generally observed that the chemical composition of the fibrous phase is closely related to that of the surrounding rock matrix.
Growth of chrysotile fibers at right angles to the walls of cracks (cross-vein) in massive serpentine formations led to the most common type of chrysotile deposit. Most of the industrial chrysotile fibers are extracted from deposits where fiber lengths can reach several centimeters (cm), but most often do not exceed 1 cm. In some occurrences, the relative motions (slipping) of blocks in the host rock, during or after fiber growth, lead to veins in which the fibers are inclined or parallel to the vein axes (slip fibers). In still other local conditions, dispersed aggregates of short fibers are found with no preferential orientation; in such cases the fiber content of the rock can be very high, up to 50%. These are called mass-fiber deposits.
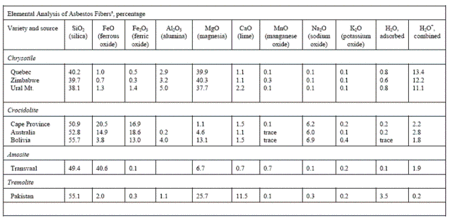
Chrysotile is a hydrated magnesium silicate and its stoichiometric chemical composition may be given as Mg3Si2O5(OH)4. However, the geothermal processes that yield the chrysotile fiber formations usually involve the co-deposition of various other minerals. These mineral contaminants comprise: brucite (Mg(OH)2), magnetite (Fe3O4), calcite (CaCO3), dolomite ((Mg,Ca)(CO3)2), chlorite ((Mg,Al,Fe)12Si8O20(OH)16), and talc (Mg6Si8O20(OH)4). Other iron-containing minerals may also be found in chrysotile, for example, pyroaurite, brugnatellite, and pyroxenes. In commercial fibers, dust particles from the host rock generated during the mining and milling processes are most inevitably present. Elemental analysis data for several chrysotile and amphibole samples are presented in the table below.
Chrysotile fibers can be extremely thin, the unit fiber having an average diameter of approximately 25 nanometers (nm) (0.025:m). Industrial chrysotile fibers are aggregates of these unit fibers that usually exhibit diameters from 0.1 to 100:m; their lengths range from a fraction of a millimeter (mm) to several centimeters (cm), though most of the chrysotile fibers used are shorter than 1 cm.
Amphiboles
 Table 1. Chemical composition of amphibole asbestos. (Source: U.S. Geological Survey)
Table 1. Chemical composition of amphibole asbestos. (Source: U.S. Geological Survey) The amphibole group of minerals is widely found throughout the Earth's crust. Their chemical composition can vary widely. Of the amphiboles, only a few varieties have an asbestiform habit and the latter occur in relatively low quantities. The geological origin of amphibole asbestos fibers appears to be quite varied. In the case of the crocidolite deposit of South Africa (Transvaal), the amphibole fibers formed during secondary chemical reactions that took place as the banded ironstone host rock consolidated from a gel of iron hydroxide and colloidal silica. Crocidolite fiber veins formed, presumably with magnetite particles acting as nucleating agents. The presence of mechanical stress appears to be necessary for the formation of crocidolite. It appears that fibers form in places where there is shearing (slip fibers) or rock dialatium (cross fiber). One would not expect fibers to form in rock that is not mechanically disturbed. The amosite deposit found in similar rock formations is the result of a high temperature metamorphic process.
The chemical composition of amphiboles readily reflects the complexity of the environment in which they formed. The average chemical composition of amphibole minerals may be represented as:
where:
- A= Na, K
- B= Na, Ca, Mg, Fe+2, Mn, Li
- C= Al, Fe+2, Fe+3, Ti, Mg, Mn, Cr
- T= Si, Al
A, B, C each represent cationic sites within the crystal structure (2, 4, 10). From this generic representation, the chemical composition of the amphibole asbestos can be given as noted in Table 1.
Chrystal Structure of Asbestos Fibers
The microscopic and macroscopic properties of asbestos fibers stem from their intrinsic, and sometimes unique, crystalline features. As with all silicate minerals, the basic building blocks of asbestos fibers are the silicate tetrahedra which may occur as double chains (Si4O11)-6, as in the amphiboles, or in sheets (Si4O10)-4, as in chrysotile.
Chrysotile
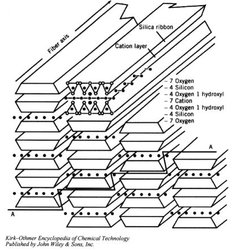
In the case of chrysotile, an octahedral brucite layer having the formula (Mg6O4(OH)4)-4 is intercalated between each silicate tetrahedra sheet. The silicate and brucite layers share oxygen atoms which would normally be separated by distances of 0.305 nm in the silicate layer and 0.342 nm in the brucite layer. This mismatch of O-O distances induces a curvature of the sheets, the ideal radius of which has been calculated as 8.8 nm. The curvature of the sheets propagates along a preferred axis leading to the formation of the tubular structure found in chrysotile. The concentric sheets forming the fibers have a curvature radius from 2.5 to 3.0 nm for the internal layers up to approximately 25 nm for the external layers, yielding unit fibers (fibrils) with external diameters ranging between 20 and 50 nm. Electron microscopy studies have also shown that, in the unit chrysotile fiber cross-section, the layers may appear in a concentric or spiral arrangement.
The stacking of the tetrahedral and octahedral sheets in the chrysotile structure has been shown to yield three types of chrysotile fibers:
- clino-chrysotile: monoclinic stacking of the layers, x parallel to fiber axis, most abundant
- formortho-chrysotile: orthorhombic stacking of the layers, x parallel to fiber
- axispara-chrysotile: two layer structure, 180° rotation of two-layer structures, y parallel to fiber axis
The extent of substitution of Magnesium and silicon by other cations in the chrysotile structure is limited by the structural strain that would result from replacement with ions having inappropriate radii. In the octahedral layer (brucite), magnesium can be substituted by several divalent ions, Fe+2, Mn +2, or Ni+2. In the tetrahedral layer, silicon may be replaced by or Al+3 or rarely Fe+3. Most of the other elements that are rarely found in vein fiber samples, or in industrial asbestos fibers, are associated with interstitial mineral phases.
Typical compositions of bulk chrysotile fibers from different locations are given in Table 3.
Amphiboles
The crystalline structure common to amphibole minerals consists of two ribbons of silicate tetrahedra placed back to back.
The plane of anionic valency sites created by this double ribbon arrangement is neutralized by the metal cations. The crystal structure has sixteen cationic sites of four different types; these sites can host a large variety of metal cations without substantial disruption of the lattice.
In contrast to chrysotile fibers, the atomic crystal structure of amphiboles does not inherently lead to fiber formation. The formation of asbestiform amphiboles must result from multiple nucleation and specific growth conditions. The difference between asbestiform and massive amphibole minerals is obvious on the macroscopic scale, although the crystalline structures of the two varieties do not exhibit substantial differences. The asbestiform amphiboles tend to have a larger number of crystal defects (Wadsley defects and twinning and chain-width disorder) than the nonasbestiform varieties. The frequency and width of these defects vary with amphibole type. Amphibole minerals, in general, are characterized by prismatic cleavage planes parallel to the c axis which intersect at an angle of about 56°. Hence, in the crushing of massive, nonfibrous amphiboles, microscopic fragments are found having the appearance of asbestos fibers. However, the statistical average of their aspect ratio is considerably lower than that of the asbestiform amphiboles.
Property of Asbestos Fibers
Asbestos fibers used in most industrial applications consist of aggregates of smaller units (fibrils). This is most evident in chrysotile that exhibits an inherent, well-defined unit fiber. Diameters of fiber bundles in bulk industrial samples may be in the millimeter range in some cases; fiber bundle lengths may be several millimeters to 10 centimeters or more.
The mechanical processes employed to extract the fibers from the host matrix, or to further separate (defiberize, open) the aggregates, can impart significant morphological alterations to the resulting fibers. Typically, microscopic observations on mechanically opened fibers reveal fiber bends and kinks, partial separation of aggregates, fiber end-splitting, etc. The resulting product thus exhibits a wide variety of morphological features (see Figure 3).
Morphological variances occur more frequently with chrysotile than amphiboles. The crystal structure of chrysotile, its higher flexibility, and interfibril adhesion allow for a variety of intermediate shapes when fiber aggregates are subjected to mechanical shear. Amphibole fibers are generally more brittle and accommodate less morphological deformation during mechanical treatment.
Fiber Length Distribution
For industrial applications, the fiber length and length distribution are of primary importance because they are closely related to the performance of the fibers in matrix reinforcement. Various fiber classification methods have thus been devised. Representative distributions of fiber lengths and diameters can be obtained through measurement and statistical analysis of microphotographs; fiber length distributions have also been obtained from automated optical analyzers.
Physico-Chemical Properties
The industrial applications of chrysotile fibers take advantage of a combination of properties: fibrous morphology, high tensile strength, resistance to heat and corrosion, low electrical conductivity, and high friction coefficient. In many applications, the surface properties of the fibers also play an important role; in such cases, a distinction between chrysotile and amphiboles can be observed because of differences in their chemical composition and surface microstructure.
Thermal Behavior
Asbestos fiber minerals are hydrated silicates so their behavior as a function of temperature is related first to dehydration (or dehydroxylation) reactions. In the case of chrysotile, the crystalline structure is stable up to approximately 550° C (depending on the heating period), where the dehydroxylation of the brucite layer begins. This process is completed near 750° C and is characterized by a total weight loss of 13%. The resulting magnesium silicate recrystallizes to form forsterite and silica in the temperature range 800-850° C, as an exothermic process. The strongly endothermic dehydration process enhances the high temperature thermal insulation properties of chrysotile asbestos.
The behavior of amphibole fibers under continuous heating is similar to that observed with chrysotile, although the temperatures of dehydroxylation and recrystallization processes are different. The amphiboles have a lower water (hydroxyl) content and their dehydroxylation reaction begins between 400-600° C, depending on the amphibole type; this reaction leads to a weight loss of approximately 2%. The products resulting from the thermal decomposition of the amphiboles are pyroxenes, magnetite, hematite, and silica.
In the presence of oxygen, the thermal decomposition of amphiboles is associated with an oxidation of divalent iron to trivalent iron, which may lead to an increase in the sample weight. The oxidation process also induces an obvious color alteration: the fibers acquire the characteristic ferric oxide color.
Asbestos fibers, in particular chrysotile fibers, can undergo substantial thermal degradation during mechanical grinding. In high-energy attrition equipment, such as ball mills, the high localized impact energies can cause the crystalline structure to become amorphous. This is particularly true for dry milling or milling in organic solvents. However, milling in aqueous slurries is less detrimental, water offering some protection against high impact degradation.
Tensile Strength
The inherent tensile strength of a single asbestos fiber, based on the strength of Si-O-Si bonds in the silicate chain, should be near 10 gigapascals (GPa) (1.45 x 106 pounds per square inch [psi]). However, industrial fibers exhibit substantially lower values, because of the presence of various types of structural or chemical defects.
The measured tensile strength of chrysotile fibers has been reported in the range 1.1-4.4 GPa (160,000-640,000 psi). The accurate determination of this parameter is difficult since the measurement performed on a fiber aggregate is influenced by interfibril adhesion, discontinuities in some of the unit fibers, mineral inclusions, etc. Consequently, higher tensile strength results are obtained for measurements done on short and thin fibers. The tensile strengths of amosite and crocidolite are comparable to that of chrysotile. With amphiboles, the tensile strength is highly influenced by the iron (Fe) content since iron-oxygen bonds located in the fiber axes, particularly those involving trivalent Fe, are particularly strong. The trend observed of increasing tensile strength of amphiboles from tremolite, to amosite, to crocidolite is directly related to the iron content of these fibers.
The variation in tensile strength of asbestos fibers as a function of temperature also sharply distinguishes chrysotile and amphiboles. Chrysotile retains (and even slightly increases) its tensile strength up to 500° C, until the dehydroxylation reaction begins; it drops sharply at higher temperatures. Amphiboles, on the other hand, exhibit a decreasing tensile strength beginning at approximately 200° C. For example, at 350° C, crocidolite has lost 50% of its initial tensile strength.
Asbestos Fibers in Aqueous Media
Although asbestos fibers cannot be viewed as water-soluble silicates, prolonged exposure of chrysotile or amphiboles to water (especially at high temperature) leads to slow progressive leaching of both their metal and silicate components. In the case of chrysotile fibers (in a given amount of water), the brucite layer will, fairly rapidly, dissolve in part, with concomitant increase in the pH of the solution. The equilibrium pH value for an aqueous chrysotile slurry reaches 10.0-10.5. Free brucite, present as contaminant in the fibers, also contributes to the pH increase.
In contact with solutions of mineral acids, organic acids, or Magnesium complexing agents, the rate of dissolution of the brucite layers is increased. When carried out to the extreme, the leaching of magnesium leads to a weight loss of 58%. The residual silica is largely amorphous and, although the fibers retain an apparent fibrous morphology, their mechanical resistance is drastically reduced.
The dispersion of amphiboles in concentrated hydrogen chloride (HCl) solutions also leads to partial leaching, the rate of which depends on the metal cations present. With crocidolite, only small amounts of magnesium and sodium are extracted in these conditions, whereas amosite liberates substantial quantities of iron and magnesium. Overall, tremolite appears to exhibit the highest resistance to acid leaching.
On the other hand, both chrysotile and the amphiboles exhibit a high degree of chemical inertia towards strong alkalies over extended periods. At high [[temperature]s], reactions with alkalies (Sodium hydroxide [NaOH], potassium hydroxide [KOH], Calcium hydroxide [Ca(OH)2]) become significant over relatively short periods; for example, crocidolite was reported to be attacked by potassium hydroxide above 100° C.
| Disclaimer: This article is taken wholly from, or contains information that was originally published by, the U.S. Geological Survey. Topic editors and authors for the Encyclopedia of Earth may have edited its content or added new information. The use of information from the U.S. Geological Survey should not be construed as support for or endorsement by that organization for any new information added by EoE personnel, or for any editing of the original content. |