Impact and abatement of acid deposition and eutrophication
Contents
Introduction/The Problem (Impact and abatement of acid deposition and eutrophication)
Acid deposition
Acid deposition (also commonly referred to as acid rain) is deposition of acidifying compounds, either dissolved in precipitation or directly in "dry" form.
The first signs of the effects of acid deposition were encountered when the loss of fish populations in Canadian, Scandinavian, and United States lakes was reported in the early 1960s. The common explanation in all cases was that the water in the lakes was acidified to a point where fish eggs could no longer produce young specimen. The cause of this acidification was acid deposition; precipitation had introduced so much acid into the lakes, that its pH had sunk below the critical limit for egg hatching.
In the mid-1980s, reports came in, specifically from Canada, the United States (see acid rain), and Germany, that unusual damage was being observed in forests. Trees had lost foliage or needles and this damage had even progressed to the point that trees died, a phenomenon which happened, for instance, in the border area of the former East Germany, Poland, and the former Czechoslovakia. In the case of tree-dieback, the cause-effect relationship was much more complicated than in the case of the loss of fish in lakes. In fact, much later, it was established that not only acid deposition, but also increased oxidant concentrations (species such as ozone, peroxy-acetyl nitrate (PAN), and hydrogen peroxide) contributed to tree damage. For an overview of tree damage in the US, see acid rain.
Eutrophication
A third type of effect of acid deposition was reported in the mid–1980s; the same deposition processes, which were responsible for loads that are too high with acid compounds, were also the cause of excessive nutrient concentrations in soil and groundwater. (A load is a time-integrated flux). This problem is called eutrophication. Nitrate and ammonium are beneficial, even essential, for development in vegetation, but in concentrations that are too high, these species lead to a diversity loss, especially in oligotrophic (adapted to low nutrient availability) ecosystems. Excessive algae growth, due to eutrophication, was causing extensive death in fish populations. Heather fields were also rapidly disappearing as the result of eutrophication. Excessive nitrate concentrations in the groundwater were measured in these same areas, making this water unfit for human consumption. This problem was mainly encountered in the Netherlands, Belgium, and parts of Germany, Denmark and southern Sweden, but it is currently also observed in the Southeastern United States, in parts of Canada, and in China.
Long-range transport
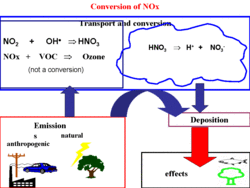
The common factor in all these cases is that pollutants, or their precursors (substances, which are converted to acidification- or eutrophication-causing compounds in the atmosphere) are emitted, transported — sometimes over long distances in the atmosphere — and deposited either by way of precipitation (wet deposition) or in dry form (dry deposition). In some cases, acid deposition takes place at distances of 1,000 km or more from the origin of the responsible emissions.
The most important acid-forming species are sulfur dioxide (which is partially converted to sulfuric acid in the atmosphere), nitrogen oxides (which are converted to nitric acid during atmospheric transport) and ammonia. Ammonia may act as a base in the atmosphere, neutralizing nitric and sulfuric acid, but ammonia in soil or groundwater is, to a large extent, converted by microorganisms into nitric acid, producing additional acid in the process.
The sources for SOx (the sum of SO2 and sulfates), NOx (NO plus NO2) and NHx (NH3 and NH4) are both natural as well as anthropogenic (human-made), see also Air pollution emissions. The sulfur compounds are eventually converted to sulfuric acid, NOx to nitric acid, and then ammonia reacts with acids to form ammonium salts. Both the precursors (SO2, NOx, and ammonia) and the products (sulfuric acid, nitric acid and ammonium) can be transported over large distances (up to 1,500 km) depending on meteorological conditions, speed of conversion, and removal by deposition processes (see Figures 1 and 2).
Wet and dry deposition are not only the processes by which pollutants are transported to the Earth's surface, exposing humans and ecosystems to acid deposition and eutrophication, but deposition processes also play an essential role in the cleansing of the atmosphere. If removal by wet and dry deposition were not to take place, the Earth’s atmosphere would be unsuitable to sustain life in a relatively short period of time (in the order of a few months).
Formation and Transport
According to EMEP (the European Convention on Long Range Transboundary Air Pollution) studies, the maximum sulfur emissions in Western Europe occurred in the period from 1975 to 1980, at a level of 40 million tons of SO2. Sulfur emissions have gone down since then and are currently (2006) at a level of about 30% of those maximum values.
In Western Europe and North America, vehicles are the main source of NOx, contributing 50% or more of the total NOx emissions in these parts of the world. Ammonia should be mentioned in the same cadre as sulfur and oxidized nitrogen compounds; one molecule of H+ is produced in the soil for each molecule of ammonia, which is converted to nitrate. The primary sources of ammonia are cattle manure and fertilizer.
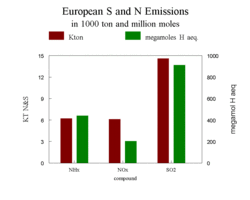
An overview of European emissions of SOx, NOx, and NHx, expressed both in kilotons and in millions of moles H+ equivalent is given in Figure 3. In terms of emitted moles or H+ equivalents (the H+ produced by these compounds), sulfur is still the dominating species, but the contribution of NHx already outweighs the NOx emissions, especially expressed as moles.
The future global development of SOx, NOx, and NHx emissions will be dominated, if emission patterns do not change, by the emissions of developing countries in South America and, especially, in East Asia. It is a reasonable assumption that Chinese NOx emissions will reach American levels within 20 to 25 years, if the economic development of China continues at its quick, present rate.
Precursors and their products are transported basically by air movements. The governing factor here is the lifetime of the different compounds. Under European conditions, species like ammonia, with a lifetime of hours, will not be transported much further than 500 km. Species like nitrogen oxides and sulfur dioxide, with lifetimes of one to three days, can be transported effectively over 1,000 to 1,500 km. Nitrate and sulfate, present as aerosols, can have lifetimes of one week and, hence, a transport range of up to 2,000 km.
Three main processes for deposition can be distinguished:
- Wet deposition, in which compounds are transported to the surface by means of precipitation, such as, rain or snow;
- Occult deposition, in which compounds dissolved in fog or cloud moisture are deposited on the surface by means of droplets; and
- Dry deposition, in which compounds are deposited to the surface directly, with no liquid phase is involved.
Wet deposition is the main deposition process in "background" areas, those regions where low concentrations of gases and aerosols occur in the atmosphere. Occult deposition is important in locations frequently exposed to fog and clouds, but it is a minor deposition mechanism in most places. Dry deposition is the dominating process in areas characterized by a high concentration of atmospheric gases and aerosols.
Wet deposition is responsible for more than 80% of the deposition load in Scandinavia, a background area. (A load is the deposition flux integrated over a time period, typically expressed in moles per hectare per year.) Occult deposition contributes more than 40% of the pollution load over Great Dunn Fell in Scotland, a hill frequently exposed to fog and [[cloud]s]. Dry deposition is responsible for more than 75% of the total deposition load in the Netherlands, which is typical for moderately polluted areas.
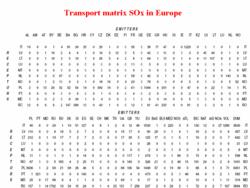
A complete overview of the import and export of acidifying pollutants for Europe was calculated by EMEP, The European Convention for Long Range Transboundary Air Pollution, and it is given in tables for sulfur compounds and nitrogen compounds (see Table 1 for sulfur compounds).
These tables provide data on the import and export of SOx for the different countries in Europe. Let’s take the Netherlands as an example. The row indicated as NL gives this information. In the first half of the table, it is indicated (see intersection of row NL and column NL) that 140×100 tons, that is, 14,000 tons of deposited SOx can be attributed to the sulfur emissions from the Netherlands itself. The contributions from Belgium, France, and Germany (see columns Be, Fr, and De) are 9,700, 5,800, and 4,200 tons, respectively. That is, less than half of the sulfur deposition in the Netherlands is emitted in that country.
Effects of Acid Deposition and Eutrophication
Aquatic bodies
As previously mentioned, acid deposition causes changes in lake ecosystems, since the hatching of fish eggs is prohibited at lower pH. Damage is also caused by free aluminum ions, because aluminum-hydroxyl complexes are converted to free aluminum ions at low pH. Free aluminum ions are toxic, not only for aquatic ecosystems, but also for forests and humans.
The impact of acid deposition on lakes is strongly dependent on the buffer capacity (that is, the capacity to neutralize acid without large changes in pH) of the lake system. This buffer capacity is a function of the composition of the surrounding soil. Lakes embedded in granite will have a very low buffer capacity, as granite weathers very slowly, and few alkaline substances are produced in the weathering process. Conversely, lakes in calcium carbonate-rich surroundings have a very large buffer capacity, because carbonate neutralizes acid.
Therefore, lakes in granite-rich areas such as Canada, Scandinavia, and Scotland, or those in sandy areas, such as some areas in the Netherlands and Belgium, are very sensitive to acid deposition.
The flux, which is equivalent to the total production of neutralizing capacity in lakes, and which would be the upper limit for a sustainable situation, is known as critical load. Some of the lakes in very sensitive areas can only be kept intact if the loads are in the order of 200 to 500 moles of potential acid per hectare per year. This is the case, for example, in southern Sweden. On the other hand, lakes in the Dolomites in Italy can easily accommodate dry deposition fluxes of 2,000 or more moles per year without any negative effects.
|
Table 2. Sensitivity of ecosystems Ecosystem Compound Limit Groundwater forest Free Al ions 2 mg/l Groundwater (human consumption) Nitrate 25 mg/l Groundwater forest Ammonium NH4+/K ratio < 5 Lakes Free Al ions 2 mg/l |
|---|
Examples of critical concentrations and ratios between different ions are given in Table 2. Seawater (Impact and abatement of acid deposition and eutrophication) has sufficient buffer capacity to be quite insensitive to acid deposition. If soil characteristics are known, the critical loads can be calculated for lakes. Excessive inputs of nutrients, nitrogen compounds in the case of deposition, lead to eutrophication in aquatic bodies. The result is an explosive development of algae populations. These large algae populations use a lot of oxygen, which can lead to low nighttime oxygen concentrations unable to support fish populations, causing asphyxiation. Another problem is the development of toxic algae, leading to botulism (high concentrations of toxic substances, produced by these algae), which renders water unfit for any use. Eutrophication is caused not only by atmospheric deposition, but also by loads of nutrients transported by rivers. The combined impact of nutrients in rivers and atmospheric deposition has led to eutrophic situations in many lakes and rivers as well as in the Baltic Sea and in certain parts of the North Sea (North Sea, Europe).
Vegetation
The direct effects of dry and wet deposition on the foliage of vegetation are important only at very high ambient concentrations. Indirect effects are generally much more important. Acid deposition leads to acidification of groundwater. If the pH of groundwater is below pH 5, hydronium ions (H+) free aluminum ions from silicates in the soil. In bounded form, aluminum is not toxic, but free aluminum ions have a very toxic effect on many species of vegetation. The effect of acid deposition is, just as in the case of lakes, dependent on the buffer capacity of the soils. In the case of granite-rich soils, this buffer capacity is very limited. The same is true for sandy soils with little carbonate. Conversely, calcium carbonate-rich soils have a very large buffer capacity. If trees are exposed to too high of a level of aluminum concentrations, less foliage is produced, and needles are lost sooner. At high concentrations, the tree becomes very vulnerable to drought and diseases. Vegetation needs nutrients, such as phosphate, nitrate, ammonium, potassium, Magnesium, calcium and sulfate. The roots of trees can, to some extent, extract ions selectively. However, if the ratio between ammonium and potassium ions is higher than a factor of 5, the uptake of potassium results also in the increased uptake of ammonium. The net result is that too much nitrogen is taken up, and “nitrogen poisoning,” occurs. Too much nitrogen leads to increased sensitivity for droughts and attacks by microorganisms and pests.
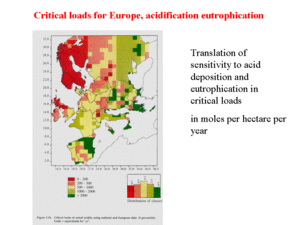
Critical limits for acid and reduced nitrogen are given in Table 1. These critical limits can be converted to critical loads for potential acid and nitrate in the case of eutrophication, using information on soil characteristics, vegetation, and land-use. As an example, an overview of the resulting critical loads for Europe is given in Figure 4. Base cations (acid-neutralizing calcium and Magnesium compounds as most important) and other neutralizing compounds can have a great influence. In some areas in China, in the Guizhou Province for instance, very high acid deposition fluxes of 10,000 moles per hectare per year or more occur, without the severe effects one would expect, based on European and U.S. experiences. It is presumed that the very high loads of neutralizing, calcium carbonate-containing dust, which are encountered in this area, mitigate the effects of these huge potential acid loads. Not only is the acid neutralized by calcium carbonate after deposition, there is also evidence that these carbonate-containing aerosols scavenge acids such as nitric acid and precursors such as sulfur dioxide. Thus, vegetation is not exposed to acids but to calcium nitrate and calcium sulfate, gypsum. Calcium nitrate is used as a fertilizer, and the soil in the Guizhou Province already contains a lot of gypsum, so the vegetation is not seriously affected at all.
Drinking water quality
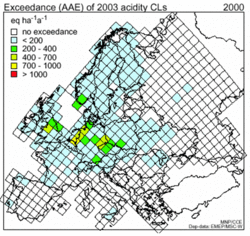
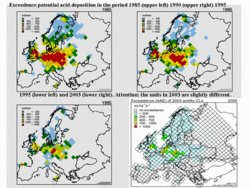
Free aluminum concentrations must be below a value of 2 mg per liter of drinking water, as aluminum is toxic for human beings as well as cattle. Nitrate is also limited; concentrations of over 50 mg per liter of nitrate in drinking water are considered to be carcinogenic. It should be noted that ammonia is, to a large extent, converted to nitrate and so contributes to the nitrate concentration in groundwater. All effects described in this article are incorporated in defining maps of critical loads in Europe. Descriptions of the soil characteristics are coupled with data on the sensitivity of ecosystems to aluminum, free acid, nitrates, and so on, and so a map of critical loads for a given area is produced. In some areas in the world (Europe is a very good example), this description of critical loads is the basis of environmental treaties under the aegis of UN-EMEP (see Figure 4). The granite masses of Scotland and Scandinavia are quite sensitive because these soils have very low buffer capacity. Whereas the soils in the mountain range of the Dolomites in Italy or the soils in the Balearic Islands in Spain consist, to a large extent, of calcium carbonate, and, hence, exhibit very large buffer capacity. This map of the critical load in Europe is compared with the "total potential acid deposition" for each of the grid cells, and the exceedance (= total potential acid deposition minus critical load) of this critical load is calculated (see Figure 5).
Abatement Based on Critical Loads
Adverse effects can be expected if the actual deposition load exceeds the critical load. The strength of these effects depends on the degree of exceedance and the length of the period in which the critical loads are transgressed. Therefore, maps of the exceedance of critical loads are the steering parameters for emission reduction measures in Europe. This exceedance, together with emitter-receptor relations, derived by long-range transport models, form the basis for the European protocols for emission abatement (Figure 5). Exceedances in the Netherlands are mainly caused by agricultural emissions, while in Germany, Poland, and the Czech Republic, they are mainly caused by industrial emissions. The exceedances in the southern parts of Scandinavia are mainly the product of the sensitivity of the ecosystems, due to the low buffer capacity of the soils. These exceedances constitute an environmental problem, but the situation was much worse 20 years ago.
The EMEP protocol, which now guides European Union abatement measures, started with sulfur emissions, but has been extended to oxidized and reduced nitrogen compounds, nitrogen oxides and ammonia. In Europe, the exceedance of the critical loads for sulfur has been reduced considerably (Figure 6). The measures taken from 1985 until the present (2006) have reduced the deposition loads quite a bit. There is still exceedance, but there is a lot less compared with earlier times, although the ultimate goal of non-exceedance has not yet been reached. However, the situation is quite different in other parts of the world, for example, in East Asia. In Europe, the abatement measures, guided by the concepts of critical loads and reduction of exceedances, have clearly been quite effective. In Figure 6, the red and orange areas, where very large exceedances of up to 1,000 moles per hectare per year occurred, have disappeared. This is a good example of the abatement policy based on a solid scientific base. This solid scientific base has been instrumental in the very general acceptance of the EU environmental policy. Measures such as fuel switch (use of natural gas instead coal), desulfurization of flue gases of coal-fired power plants, low-nitrogen oxide-emitting gas and oil burners, car exhaust catalysts and low-emission application of fertilizer have contributed very significantly.
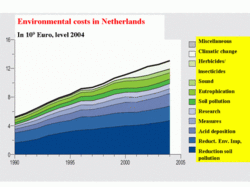
There are uncertainties in critical loads, considerable gaps in our knowledge about emissions and deposition of reduced nitrogen compounds, etc., which will require additional attention. All in all, a 50% to 75% uncertainty factor, or more, must be assumed in the exceedance estimates. This is not a problem if very large exceedances (for example, 2 or 3 times the critical loads) are present. However, these uncertainties are problematic as the exceedance decreases, which is the case right now in Europe. The cost of abatement measures generally rise exponentially with the amount of reduction. Therefore, small marginal reductions in the present levels of abatement in Europe will cost a lot of money. But, for other places this kind of uncertainty is not important, since abatement measures, as well as exceedances, are large. This problem can be derived from Figure 7, which gives the total costs for environmental measures in the Netherlands. About 2 billion Euros out of a total of 12 billion Euros — which for a country of 16 million inhabitants and a 30,000 square kilometer area is a very large sum — is devoted to the abatement of acid deposition.