Acid Deposition
Contents
Acid deposition fact sheet
This ecology fact sheet was originally published by the Ecological Society of America (ESA). This fact sheet may be updated periodically; for the latest version, please see the ESA website.
Click here (Acid deposition fact sheet) to see more fact sheets from the Ecological Society of America.
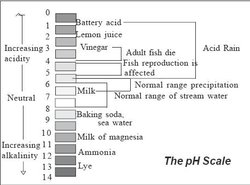
During the early 1980s and 90s, dramatic visions of acid precipitation corroding buildings and killing trees captured the minds of the public. Since then, the threat of “acid rain” has lost some of its celebrity appeal, due in part to government policies aimed to mitigate the problem and environmental issues, such as global warming, that have taken center stage. But acid deposition continues to affect ecological systems and is likely contributing to forest degradation, fish kills, and tainted water quality. But what exactly is acid deposition and where does it come from? What are its short-term and long-term ecological effects?
What is Acid Deposition?
Acid deposition, commonly known as acid rain, occurs when emissions from the combustion of fossil fuels and other industrial processes undergo complex chemical reactions in the atmosphere and fall to the earth as wet deposition (rain, snow, cloud, fog) or dry deposition (dry particles, gas). Rain and snow are already naturally acidic, but are only considered problematic when less than a pH of 5.
The main chemical precursors leading to acidic conditions are atmospheric concentrations of sulfur dioxide (SO2) and nitrogen oxides (NOx). When these two compounds react with water, oxygen, carbon dioxide, and sunlight (Solar radiation) in the atmosphere, the result is sulfuric (H2SO4) and nitric acids (HNO3), the primary agents of acid deposition.
Airborne chemicals can travel long distances from their sources and can therefore affect ecosystems over broad regional scales and in locations far from the sources of emissions.
|
A variety of ecological, chemical, physical, and human factors determine the vulnerability of an ecosystem to acid deposition:
|
 Fossil fuel burning creates sulfur and nitrogen emissions. (Courtesy of the US Geological Survey; Source: ESA) Sulfuric Acid Deposition Sulfur is stored in various organic substances such as coal, oil, and peat. These long-term pools of stored sulfur are released into the air as a result of fossil fuel combustion and natural phenomena such as volcanic eruptions. Sulfur dioxide in the atmosphere, which is converted to sulfuric acid, is identified as one of the major causes of acid deposition. Human sources of SO2 emissions: The primary sources of human created sulfur dioxide include electric utility and power plants that burn coal and oil products. Automobile exhaust also contributes to SO2 emissions. In 1992, the U.S emitted 22.7 million tons of SO2 into the atmosphere. Government policies have helped to reduce these emissions. Between 1995-1998, total national SO2 emissions averaged 19.6 millions tons/year, nearly a 14% reduction from 1992 levels. Nitric Acid Deposition Nitrogen exists in several different forms and is an essential element to plant and animal growth. Nitrogen is only considered a pollutant when it exists in high enough concentrations to cause acid deposition or ecosystem eutrophication (excessive nutrient enrichment). Nitrogen in the form of nitrogen dioxide (NO2) is very reactive in the atmosphere, becoming nitric acid which is a key contributor to acid conditions. Human sources of NOx emissions: The primary human source of nitrogen that is emitted to the atmosphere comes from the combustion of fossil fuels for transportation, electric utilities, and industrial processes. In 1997, the U.S released 23.8 tons of NOx into the atmosphere, and these emissions are expected to increase with growth in electricity demand and vehicle miles traveled. However, current government regulations and changes in industrial practices are expected to help reduce the rate of these emissions from the electricity sector over time. |
What are the ecological effects of acid deposition?
Acid deposition may cause significant stress to lakes, streams, and forest ecosystems, especially to those at higher elevations. Acidification is classified in two forms:
- Episodic acidification is characterized by short intense acidic events. For example, winter snowmelt and heavy [[rain]s] can deliver large loads of acid to ecosystems in a short period of time. These can have significant biological effects that include the loss of biodiversity and changes in community structure.
- Chronic acidification generally refers to streams, lakes, and soil ecosystems that have lost their ability to neutralize acidifying events. Base nutrients such as calcium, potassium, and Magnesium, and other types of neutralizing chemicals buffer changes in ecosystem acidity. However, when ecosystems are exposed to excessive, long-term acid deposition these chemicals become depleted. This can make the system more vulnerable to episodic acidification events and may lead to chronic surface water acidity.
Ecological Impacts on Soil, Forest, Freshwater, and Estuarine Ecosystems

- Base Nutrient Depletion: Acidification of soils causes the removal of nutrients (a process known as leaching) from terrestrial ecosystems. These nutrients, known as base cations, are critical in neutralizing acids. As forest soils lose nutrient cations, they become more vulnerable to further acidification, and, as a result, trees become more sensitive to disease and stress. An example of this occurs in western Pennsylvania where a high rate of sugar maple and red spruce mortality is blamed on the depletion of nutrient cations caused by chronic acidification.
- Aluminum Toxicity: The release of the heavy metal aluminum from the soil due to increased acid deposition can create unsuitable living conditions for many freshwater (Freshwater biomes) fish species. High concentrations and extensive exposure of lakes and streams to aluminum is a main symptom of systems suffering from chronic acidification.
- Nitrogen Saturation: Nitrogen saturation occurs when excessive additions of nitrogen overwhelm an ecosystems’ capacity to store nitrogen. Since nitrogen is a nutrient normally in limited supply in forest and estuarine ecosystems, the addition of large amounts of nitrogen from nitric acid deposition can have significant ecological effects. At first, increased nitrogen inputs may stimulate forest growth, but too much nitrogen in ecosystems overwhelms the holding capacity of these systems and eventually causes soil, forest, and aquatic ecosystem degradation.
- Eutrophication (nutrient enrichment): When nitric acid enters terrestrial and aquatic systems from the atmosphere, a portion of this added nitrogen eventually reaches coastal areas, with the potential to create eutrophic conditions. Increased inputs of anthropogenic (human) nitrogen have caused harmful impacts on coastal ecosystems that include hypoxia/anoxia (low oxygen levels), fish and shellfish kills, and changes in algal community composition. Biodiversity in coastal ecosystems can be greatly threatened under these ecologically-stressed conditions.
 Acidification causes forest health decline. (Courtesy of the US Geological Survey; Source: ESA) In the U.S., the ecological effects of acid deposition vary from region to region. In the eastern U.S., ecological damage from acidification (nitric and sulfuric acid deposition) is widely apparent in the Adirondack and Catskill Mountains of New York. Many lakes and streams in these areas are no longer able to maintain fish populations, and water quality in these areas has been highly degraded. Similar situations are also reported in the Smoky Mountains, Shenandoah National Park, and in several midwestern states. Eastern Canadian ecosystems have also been similarly hard hit by acid deposition. In the western U.S., nitrogen leaching in high elevation ecosystems has just recently been detected in the Colorado Rockies Front Range. In contrast, the rate of nitrogen leaching into streams surrounding chaparral [[forest]s] of the Los Angeles Basin, CA, is one of the highest recorded in the country. Nitric acid deposition is of greater concern in the West than sulfuric acid. In some coastal ecosystems deposition of nitric acid is a key contributor to poor water quality conditions and massive fish kills. Outside the U.S., scientists predict that future NOx and SO2 emissions will substantially increase in the developing world, especially in Asia, making acidification a greater ecological problem. |
 (Source: ESA) Our understanding of atmospheric pollution (Air pollution emissions) and its effects on the environment has increased over the last decade and has provided scientists with fresh perspectives and policy makers with new approaches for dealing with the problem. However, ecologists are still trying to fully understand the long-term effects of acid deposition on ecosystems. This requires long-term studies that include an array of ecosystem types over decades rather than years. For instance, there is still much to learn about the impacts of acid deposition on soils, surface water chemistry and biology, [[forest]s], and ecological response and recovery. The role of nitrogen and its cycling (Nitrogen cycle) through ecosystems is also a significant area of current research. Long-term monitoring (Environmental monitoring and assessment) is a critical component of scientific research. This type of data contributes important information to scientists who study acid deposition. Chemical and biological monitoring enable researchers to evaluate the effectiveness of policies intended to reduce air pollutants as well as to test scientific models of ecosystem responses to acid deposition. |
For More Information
Other ESA publications on acid deposition include:
- Fact sheet, “Acid Rain Revisited”, a policy overview
- Workshop report, “Acid Deposition: The Ecological Response”
- Workshop report, “Atmospheric Nitrogen Deposition to Coastal Watersheds”
- Workshop report, “Where Air and Water Meet: The Role of Atmospheric Deposition in the Gulf of Mexico Hypoxic Zone"
- Issues in Ecology #1, “Human Alteration of the Global Nitrogen Cycle: Cause and Consequences”
Other sources of information:
| Disclaimer: This article is taken wholly from, or contains information that was originally published by, the Ecological Society of America (ESA). Topic editors and authors for the Encyclopedia of Earth may have edited its content or added new information. The use of information from the Ecological Society of America (ESA) should not be construed as support for or endorsement by that organization for any new information added by EoE personnel, or for any editing of the original content. |