Abiotic factor
An abiotic factor is any of a number of the non-living components of a habitat. Abiotic factors can be grouped into the categories of meteorology, soil, air pollution, micro-topographic features, water availability and water quality. In terms of meteorological factors, the primary abiotic factors can be construed to be temperature, precipitation, wind velocity, solar insolation and humidity. It should be noted that statistical variation and seasonal variation of these basic parameters can be important elements of the habitat description as well as the temporal correlation of these variables; for example, for certain amphibian species, it is not only the average annual rainfall which is important to reproductive success, but especially the timing of rainfall that occurs in breeding season or the rainfall that occurs within the temperature optima for breeding. In addition the thawing timing of ponds can also be significant.[1]
Edaphic or soils factors include variables such as soil granularity, soil chemistry and nutrient content, as well as nutrient availability. These factors are made more complex in that there may be interactions of the appropriate concentrations of minerals or nutrients with the timing of precipitation; furthermore, the vertical profile of soil chemistry can also be significant.
Air pollution factors can be significant for both plants and animals. In the case of fauna, the presence of such gases as carbon monoxide and sulfur dioxide can lead to degradation of circulatory or pulmonary function, and for high and prolonged concentrations, even death. In the case of vascular plants, air pollutants can enter into stomatal openings; upon such penetration, many air pollutants can impair metabolic function, particularly photosynthesis. Air pollutants can also damage leaf, stem and flowering structures. For lichens the interferences with metabolism can be even more sensitive, since most chemical uptake is via air; for example, in the case of certain lichens, there is little tolerance to excesses of air pollutant concentration. The sensitivity of a lichen to air pollution is directly related to the energy needs of the mycobiont, so that the stronger the dependency of the mycobiont on the photobiont, the more sensitive that lichen species is to air pollutants.[2]
Contents
Meteorology
See main article: Meteorology
Meteorologcal factors can strongly influence the functioning of an ecosystem. Even though large scale proceesses of the atmosphere (Atmosphere layers) involve interactions with the Earth's crust, oceans and outer space, microscale meteorology is an inherent part of any terrestrial or aquatic ecosystem. The chief meteorological parameters that comprise abiotic factors of ecosystems are temperature, sunlight, wind velocity, barometric pressure, humidity and the gradients and interactions of each variable, as well as their temporal variability. Meteorology and hydrology compose the interdisciplinary field of hydrometeorology, which is also a significant set of abiotic factors. Meteorological abiotic factors may be simply the prevailing climatic features that define an ecosystem's atmospheric (Atmosphere layers) abiotic features; in some cases, the meteorogical factors may be episodic or even catastropic events that define major transformations of an ecosystem. Examples of such abiotic upheavals are windtrhow from hurricanes and tornadoes; torrential floods that scour and uproot large amounts of vegetative cover; prolonged drought which may alter the plant association and animal ecology.
Soil
See main article: Soil
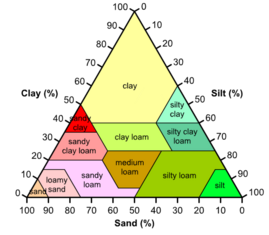
In a broad sense soil should be considered to be not only the mineral components commonly deemed inherent in the name, but also air, water and even dead organic material; however. since air and water are treated as separate abiotic factors in this treatment, only the mineral and geometric aspects of soil will be discussed here. Moreover, the dead organic material can be considered as a component of the soil, since it is not living, even though organic in origin. Soil may be considered thus as a complex variety of mineral particles plus the dead organic matter. It is also important to consider the voids within soil as a property of the soil, since the packing density and shape of soil particles affect the resulting characteristics of water and plant root penetration, as well as the hosting of organisms from micro-organisms to large animal burrows.
Technically soils can be as impermeable as solid non-porous rock, or as highly pervious as coarse sand. The granularity of soils is generally merely a function of the geologic time weathering of the local earth crust as well as the depositional history of fluvial, marine and aeolian processes. The resulting soil permeability plays an important role in determining the plant palette that can adapt to a given habitat. Loosely packed or highly pervious soils generally are poor in near surface water retention, but effective in encouraging downward percolation of water, with the result of enhancing local groundwater basins and thus sustaining water supply in the wider basin. Such loose soils are also hospitable to root penetration and thus plant growth, provided that rainfall or runoff is sufficient to supply the needs of the plants in an environment of marginal surface soil water retention. The coarsest such soils are gravels, which may be quite ineffective in supporting plant growth, if there no intervening finer soil particles.
In the opposite extreme of dense closely packed soils, one may see such examples as solid rock or hardpan clay. Such extremes in impervious soils are not supportive of plant life, since downward percolation of water is impeded and high surface runoff (Surface runoff) is encouraged. On the other hand, hardpan soils may foster longer term retention of surface waters that manage to accumulate, in the case of level terrain, micro-depressions or high precipitation environments. An example of such a specialized habitat is the vernal pool[3] which manifests strong seasonal variations of plant growth and the encouragement of flora specialists to this dramatic variation in surface soil moisture. The presence of large quantities of dead organic material typically enhances the water retention of surface soils, and generally provides more hospitable growing environments for plants,[4] as well as water storage for many faunal species. In any case the soil texture and dead organic content play a key role in determining not only the plant association but also the life support system for animals in a given habitat.
Air pollution
See main article: Air pollution
The term air pollution is applied here, since the majority components of air (nitrogen, oxygen, and carbon dioxide) do not typically have great variability over large spatial regimes and hence are not important habitat determinants. On the other hand, sulfur dioxide, carbon monoxide, reactive hydrocarbons, oxides of nitrogen, heavy metals, particulate matter and other man-produced chemicals have considerable spatial variability and hence can play a key role in determining the outcome of plant association and faunal fitness in a given habitat.
Certain chemicals such as sulfur dioxide have potent adverse impacts upon both vegetative metabolism as well as animal health. Commonly occurring localized levels of sulfur dioxide from man-made sources can readily reduce plant productivity by about 30 to 50 percent,[5] and it can severely adversely affect respiration function, metabolism and mortality of many faunal species, including humans. Many molecular gases can actually enter the stomatal openings of plants and directly interfere with photosynthesis; particulate matter, on the other hand can clog stomatal openings and reduce the gross intake of carbon dioxide by plants.
In the case of heavy metals, the pathway of impact to the ecosystem is typically deposition to soils and subsequent uptake by plant roots. Cadmium, for example, is highly toxic to most plants at concentrations as low as three parts per billion in soils.[6] Presence of heavy metals in soils thus can severely inhibit plant development and effectively reduce the diversity of the plant palette. Correspondingly herbivores and carnivores higher in the food chain can concentrate such trace elements, with generally adverse results to animal fitness and reproduction.
Topographic factors
See main article: Topography
Micro-topography can have important influences upon habitat definition, both as to adaptations of plants and animals, as well as bacteria and other organisms. With respect to plantlife, topography interacts with meteorology in producing a variety of wind shear, turbulence, and thermocline effects that can influence plant growth and even plant selection for a given habitat. Topography interacts with soil type by influencing the ratio of surface runoff (Surface runoff) to downward percolation following precipitation; in fact, micro-topography shapes the fundamental ponding that leads to surface water retention and vernal pool formation, factors significant in determining plant viability and selection.
With respect to animal life, topography influences the suitability of habitat for burrows,[7] for nests, for hiding from predators (and conversely for stalking by predators) and for transport efficiency with respect to animal movement capability (speed and traction). As nesting examples, certain birds have a clear preference for cliffside nesting sites, requiring extreme verticality in micro and macro topography; puffins and many penguins have a slope preference for their burrows, which slope is somewhat dependent on the exact soil type. An example of movement restriction, migrating salamanders have a maximum slope tolerance for micro-topography, which is also soil type dependent.
Water availability
Besides the consideration of water introduced by precipitation or condensation, the availability of water throughout a habitat is a fundamental determinant of what plants and animals can adapt locally. The chief water availability parameters are controlled by soil and topography, but deserve discuss as a separate topic due to the importance of water availability. Thus the subject of water availability can be defined by the issues of surface runoff (Surface runoff) characteristics, downward percolation, water retention in the upper soils,[8] evapotranspiration, groundwater flows and surface water characteristics. At an operational level the key factors are pathways of water availability to plants and storage/timing of water availability to animals. The extreme conditions of aridity or flooding define special habitats, which are respectively deserts or wetlands.
Water quality
See main article: Water pollution
As important as water availability is the quality of water within a habitat. This topic embraces not only concentrations of chemicals present in natural water systems, but also to human introduced chemicals. Significant naturally occurring constituents include nutrients and trace minerals used in organism metabolism; among nutrients, nitrate, phosphate and potassium are some of the most fundamental ions taken up by plants and animals. With regard to man-produced pollutants, some of the chief components are petroleum hydrocarbons, pesticides, herbicides and heavy metals. Trace minerals that are often important to metabolic function include zinc, Magnesium and iron; each trace mineral that is beneficial to organisms can be classed as a pollutant if human produced discharges to the environment accumulate to a high level. In many cases heavy metals and complex organic materials may accumulate in plant and animal tissue, subsequent to uptake from the envrionment.
Organisms adapted to high aquatic mineralization conditions are examples of extremophiles; brine shrimp (genus Artemia) and brine flies (genus Ephedra) are genera with such specialized habitat requirements. Lake Urmia in Iran and Mono Lake in California are locations where such hypersaline lake habitats are found.
One of the earliest mathematical models addressing chemical dissolution in runoff (Surface runoff) and resulting transport was developed in the early 1970s under contract to the United States Environmental Protection Agency (EPA).[9] This computer model formed the basis of mitigation research that led to strategies for subsequent land use and chemical handling controls.
pH
See main article: pH
pH is actually a component of water quality, but it is sufficiently important to be treated as a separate parameter within the abiotic factors.[10] The pH of water is the concentration of hydrogen ions (H+) in an aqueous solution; it is also a usedl to measure the acidity or base level of soil. The lower case p in pH stands for "power of" with H being the symbol for the element hydrogen. Mathematically, it is the negative log of the concentration in molarity of hydrogen ions in a solution. For chemists, the term hydronium ion (H3O+ ) is often substituted for hydrogen ion.
Water undergoes dissociation into hydrogen ions (H+) and hydroxyl ions (OH-). When the concentrations of these two ions are equal, the solution is considered neutral. If the concentration of hydrogen ions is larger than the concentration of hydroxl ions, the solution is acidic. If hydroxyl ions are in greater concentration, the solution is considered alkaline or basic. Pure water at room temperature will have a neutral pH of 7.00. Values of pH below 7.00 are found in acidic solutions while values above 7.00 characterize basic solutions.
The pH of natural rainwater is slightly acidic, around 5.6, because carbon dioxide dissolves in water and forms carbonic acid. Human blood has a pH of 7.3-7.5. Seawater has a pH of 7.8-8.3
Each organism has an optimum range of pH tolerance, since pH governs most basic metabolic processes within cells, as well as molecular transport through cell walls. The pH of a solution controls mineral solubility, solubility and structure of organic molecules, and protein structure.