
|
Maize
Scientific name:
Zea mays
Order/Family:
Cyperales: Poaceae (Graminae)
Local names:
Mahindi (Swahili); Mbembe (Kenya)
Pests and Diseases:
African armyworm
African bollworm
African maize stalkborer
Angumois grain moth
Aphids
Common rust
Common smut
Couch grass
Cutworms
Ear rots
Grasshoppers
Grey leaf spot
Head smut
Larger grain borer (LGB)
Maize ladybird beetle
Maize leafhoppers
Maize plant hopper
Maize streak virus
Northern leaf blight
Purple witchweed
Satintail
Sedges
Southern leaf blight
Southern rust
Spider mites
Spotted stemborer
Storage pests
Termites
White grubs
|
The African armyworm (Spodoptera exempta)
The African armyworm is a very damaging pest, capable of destroying entire crops in a matter of weeks. Although they are regarded as occasional pests, in an outbreak large number of caterpillars will appear destroying the whole plant to ground level.
- Monitor regularly field margins, low areas where plants have lodged, beneath plant debris around the base of plants, on the ground, and underneath the plant leaves. Check daily young crops if conditions are known to be favourable to the pest.
- Spray Bt or botanicals such as neem and pyrethrum extracts. Spray when caterpillars are small. Once caterpillars are mature (about 3 to 3.5 cm long) they may have already caused serious damage and it may no longer be economical to treat the crop. For more information on (neem click here, for pyrethrum click here and for Bt click here)
- Conserve and encourage natural enemies. For more information on natural enemies click here
- Practise field sanitation. For more information on field sanitation click here

© University of Arkansas
African bollworm (Helicoverpa armigera)
Caterpillars of the African bollworm also known as the corn worm or earworm attack mainly the developing cobs, although they may occasionally feed in the leaf whorl or on tender tassels. Eggs are laid on the silks. Caterpillars invade the cobs and feed on developing grain. Development of secondary infections is common. Local outbreaks of this pest are sometimes severe.
- Conserve natural enemies. Parasitic wasps, ants and predatory bugs are important in natural control of the African bollworm.
- Monitor the crop regularly.
- Use bio-pesticides. Plant extracts (e.g. neem, garlic, chilli,) and Bt are reportedly effective against the African bollworms. However, timing of application is very important. Spraying when caterpillars are inside the cob would be ineffective. For more information on neem click here. For information on Bt click here
- Handpick and destroy pod borers. This helps when their numbers are low and in small fields.

© A.M. Varela, icipe

African bo…

African bo…
Stemborers: African maize stalkborer (Busseola fusca)
Stemborers are the most important insect pests of maize in sub-Saharan Africa. Yield losses vary between 10-70%. Several species have been reported. The importance of a species varies between regions, within a country or even the same eco-region of neighbouring countries. At least four species attack maize in eastern and southern Africa, with yield losses reported to vary from 20 to 40%, depending on agroecological conditions, crop cultivars, agronomic practices and intensity of infestation.
The most important are the African maize stalkborer (Busseola fusca) and the spotted stemborer (Chilo partellus) (see also below).
The pink stalkborer (Sesamia calamistis) and the sugarcane stalkborer (Eldana saccharina) are of minor importance in maize.
Early warning signs: Young plants have pinholes in straight lines across the newest leaves. This is the time to treat - before the caterpillars move into the stem.
- Conserve natural enemies. Parasitic wasps and predatory ants are important in natural control of stemborers.
- Destroy crop residues to kill pupae left in old stems and stubble and prevent carry-over populations. This helps in limiting initial establishment of stemborers on the following season's crops.
- Intercrop maize with crops that are non-hosts for stemborers (e.g. cassava and grain legumes).
- Intercrop maize with a repellent plant such as desmodium and plant an attractive trap plant, such as Napier grass, as a border crop around this intercrop to protect maize from stemborers. This technology is known as "push-pull". For more information on push-pull click here
- Use neem products. Simple neem products are reported to be effective for control of stemborers. Place a small amount of neem powder (ground neem seeds) mixed with dry clay or sawdust at a rate of 1:1 in the funnel of the plant. One kg powder should be sufficient to treat 1500 to 2000 plants. Rainwater dissolves the active substances in neem powder as it gathers in the funnel and washes out the powder. Where rainfall is irregular a liquid neem seed extract can be sprayed into the funnel.

© A. M. Varela, icipe

African ma…

African ma…

Sugarcane …
The Angoumois grain moth (Sitotroga cerealella)
The larvae of the Angoumois grain moth penetrate and feed inside maize grain. This insect may also infest the crop in the field prior to harvest. The moths are small (nearly 1 cm long) yellowish or straw-coloured, a have a fringe along the posterior margins of the wings. They can be observed flying around infested stores.
Female moths lay eggs at night. Eggs are laid singly or in clumps on the outside of cereal grains, in cracks, grooves or holes made by other insects. Eggs are initially white turning red near hatching. The larvae are whitish. The larvae prepare a round exit hole for the moth, leaving the outer seed wall only partially cut as a flap over the hole, resembling a trap door. The adult pushes its way out through this "window" leaving the trap door hinged to the grain. Infested grains can be recognised by the presence of these small windows. The adult lifespan may be up to 15 days, and 1 female can lay over 100 eggs.
- Practise store hygiene. All residual pockets of infestation should be cleaned out at the end of the storage season. This is important to minimise re-infestation of the new crop.
- Store old and new lots separately.
- Do not leave maize in the field after drying, this increases the chances of infestation.
- Whenever possible separate stores from fields. The grain moths are good flyers and adults from infested stores often infest growing maize in the field.
- Keep the temperature and humidity as low as possible. There are indications that storing grain in a dry place can reduce infestation.
- Prevent pest entry by sealing the store (windows, doors, ventilation facilities) with insect-proof gauze. Hermetic storage at low humidity gives good levels of control. In Malawi, plastering stores with mud to reduce water uptake was found to be effective (Golob and Muwalo, 1984, CABI, 2000).
- Periodically inspect and remove any infested maize.

© Clemson University- www.insectimages.org
The maize aphid or corn leaf aphid (Rhopalosiphum maidis)
It is dark green to bluish-green in colour with black cornicles. Particularly during dry/periods the colonies appear on the inflorescences and young leaves. Feeding by this aphid causes yellow mottling, but this damage is seldom of economic importance. Their role as vector of the sugarcane virus, maize dwarf mosaic virus and maize leaf-fleck virus makes them a pest of considerable importance. This aphid usually attacks maize plants at the end of the mid-whorl stage. Aphid colonies may completely cover emerging tassels, and the surrounding leaves, preventing pollen release. In severe outbreaks the ear shoot is also infested, and seed set may be affected.
- Conserve natural enemies. Aphids have a wide range of natural enemies, which normally keep them under control.

© www.inra.fr

Maize aphi…

Aphids

Maize aphi…
Common rust (Puccinia sorghi)
It is recognised by the appearance of circular to elongate pustules scattered over both surfaces of the leaf. Pustules are powdery and cinnamon-brown in colour. They contain masses of spores (uredospores). Pustules can appear on any above-ground part of the plant, but they are most abundant on the leaves. With time the pustules split exposing the spores, which are spread by wind and initiate new infection. As maize matures, colour of spores in pustules change from reddish to black due to formation of teliospores (resting spores). The disease is spread by air transport.
- Use of resistant varieties if available.
- Deep plough crop residue.
- Destroy the weed Oxalis sp. (an alternate host).

© Courtesy EcoPort (http://www.ecoport.org): David C. Nowell
Common maize smut (Ustilago maydis)
Characteristic symptom of common smut is formation of galls or tumuors on above-ground parts of maize plant. Galls frequently are from one to several centimetres in diameter. The galls are at first covered by a shining, whitish-green membrane. As the gall enlarges, the membrane ruptures, exposing a powdery black mass of spores.
- Use resistant varieties if available.
- Practise crop rotation.
- Practise field sanitation. In smallholdings remove and destroy smut galls before smut spores are produced. This may help reduce prevalence of the disease in following years.

© Courtesy EcoPort (http://www.ecoport.org) : Grahame Jackson
Couch grass or Bermuda grass (Cynodon dactylon)
It is a spreading perennial grass with vigorous mat-forming stolons. It reproduces and spreads mostly by means of rhizomes but also propagates by seed. This grass is considered as one of the most important weeds in the world. It is present in virtually every tropical and subtropical country and in virtually every crop in those countries. Couch grass and other species of Cynodon are common in East Africa, and some species are occasionally troublesome as a weed of arable land and perennial crops.
Couch grass is reported in Ghana as a problem in crops such as eggplant, okra, onion, peppers and tomato.
- Where couch grass is a problem, control it before planting maize, as it will not be possible to grow a profitable maize crop in a couch dominated field.
- Harrow with a tooth harrow during the dry season in order to uproot the rhizomes and letting them dry completely on top of the soil. If possible, collect and burn dry rhizomes. Burning them will increase the success of couch control.
The same tooth harrow can be used to sweep the dry rhizomes together in bands on the field which can then be burned on site or collected and used for fuel elsewhere (farmer experience). - Introduce shade producing cover crops, within a crop rotational system.

© Charles T. Bryson, USDA ARS, www.insectimages.org

Couch gras…

Couch gras…

Couch gras…
Cutworms (Agrotis spp. and other species)
Cutworms cut maize seedlings at or a little below ground level, make small holes along the initial leaves, or remove sections from the leaf margins.
- Eliminate weeds early, at least 2 weeks before transplanting.
- Plough and harrow the field prior to transplanting. This exposes cutworms to natural enemies and desiccation and helps destroy plant residue that could harbour cutworms.
- Make barriers to protect the transplanted seedlings. Barriers can be made by wrapping paper, aluminium foil, thin cardboard or similar materials around the base of transplant stems. Toilet rolls are handy as cutworm collars since they are readily available and will biodegrade into the soil.
- Dig near damaged seedlings and destroy cutworms.
- Conserve natural enemies. Parasitic wasps and ants are important in natural control of cutworms.

© A.M. Varela, icipe
Ear rots (Gibberella zeae / G. fujikuroi)
Characteristic symptoms include pink to brick-red colour on ears, husks and kernels. The fungi often gain entrance to the ears through channels made by earworms and borers. Bird damage to the ears also facilitates disease infection.
Symptoms
Roots: dry rot.
Seedlings: blight and subsequent death of the seedling.
Leaves: leaves become a dull green colour when rots and stalks are infected early.
Stalks: lesions are a dark brown to black colour in which black perithecia may be produced near the lower nodes. The pith is shredded and is pink to red in colouration.
Ears: the fungus infects the ear via the silk channel and causes a red rot of the kernels from the tip of the ear. This may spread over the whole ear.
- Use resistant varieties if available
- Manage pests attacking the ears.
Grasshoppers and locusts
Several species of grasshoppers and locust feed on maize. The edible, a long horned grasshopper Homorocoryphus nitidulus vicinus (Ruspolia differens) has been reported to occasionally attack maize in Tanzania (Bohlen, 1973). This grasshopper attacks maize in the silking stage, arresting pollination. Other grasshoppers and locust attack maize from the mid-whorl stage to maturity, and may consume every part of the plants. Attacks vary in severity from location to location.
- Conserve natural enemies. Avoid destroying larvae of blister beetles, since they feed on eggs of grasshoppers. Other natural enemies include ants, parasitic flies, assassin bugs, predatory wasps, birds, lizards, snakes, frogs, and fungi. Robber flies are a major predator of grasshoppers.
- Domesticated poultry (e.g. chickens, turkeys, guinea fowl, geese, and ducks) and wild birds are good for keeping grasshopper populations in check. However, birds may damage the plants too. To avoid this enclose the birds in wire fencing along the perimeter so that they can prey on visiting grasshoppers while staying out of the crop.
- Ensure the ground is covered with crops, grass or mulch. This is reported to reduce grasshopper numbers since they prefer laying eggs on bare soil.
- Catch grasshoppers by hand or with a butterfly net. Catching them in the early morning is easier, as they are less active in the mornings.
- Dig or cultivate the land before planting to expose the eggs to predators and to the sun.
- Whenever necessary spray biopesticides. Neem extracts act as antifeedant (grasshoppers stop feeding when exposed to neem products) and affect development of grasshoppers. For more information on neem for control of grasshoppers link to section of grasshoppers in cassava datasheet. IITA (the International Institute of Tropical Agriculture) researchers and partners have developed an environmental friendly biopesticide "Green Muscle" based on a naturally occurring fungus strain indigenous to Africa (Metarhizium anisopliae). This fungus is deadly to locusts and grasshoppers but reportedly does not damage plants, animals- or people. Typically 70 to 100 percent mortality rates were obtained after 8 to 28 days of application. (www.iita.org)

© Kurt Kulac
Grey leaf spot (Cercospora zeae-maydis
Symptoms are similar to Southern leaf blight but the spots are much narrower. They are initially light brownish in colour, and with age they bleach to ashen grey surrounded by narrow light-brownish border. When wet, spore mass is formed on the spots with a light shade. This disease is favoured by prolonged periods of high relative humidity. It can cause yield losses of 30 to over 50%.
- Use resistant varieties if available.
- Practise field sanitation. It helps in reducing the inoculum (infection) source.

© A.A. Seif, icipe
Head smut (Sphacelotheca reiliana)
The first symptoms become evident when tassels and cobs (ears) appear. These parts may be completely or partly converted into smut galls. Smut galls are initially covered by a delicate membrane that breaks open and exposes a mass of reddish-brown to black spores and strands of vascular tissue. The strands or fibres in the galls distinguish this disease from common smut. Head smut is seed-borne.
- Use resistant varieties, if available.
- Practise crop rotation.
- Eliminate volunteer host plants.

© Courtesy EcoPort (http://www.ecoport.org): David C. Nowell
The larger grain borer (Prostephanus truncatus) and the grain weevils (Sitophylus spp.)
They attack stored maize grains. Both the adults and the larvae (grubs) of these beetles feed in the grains. Adults come from infested cobs in the field or from an infested maize store and lay eggs in the grains. They attack maize both in the field and after harvest. Attacked maize grains lose all their contents and are not fit to eat. These pests become a serious problem in short time if no control measures are applied. The larger grain borer also attacks dried cassava roots and even the wooden structures of the stores.
- Conserve natural enemies. An imported predatory beetle Teretrius (formerly Teretriosoma) nigrensis has been released in several African countries in an attempt to control the larger grain borer.

© NRI/MAFF. Reproduced from the Crop Protection Compendium, 2004 Edition. © CAB International, Wallingford, UK, 2004

Larger gra…

Maize weev…
The maize ladybird beetle (Epilachna similes)
The adult is oval in shape, about 6 mm in length and reddish brown in colour with black spots on the wing covers. The body is covered with short, light coloured hairs. The larvae are 7-9 mm in length, soft and covered with dark coloured spines. They pupate on leaves. Both larvae and adults of the maize ladybird beetle feed on leaves, scrapping them, usually on the underside, leaving the upper epidermis intact. This beetle will cause much damage only when present in large numbers. It also attacks cereals such as wheat and sorghum.
The maize ladybird beetle rarely causes serious defoliation and therefore control is usually not necessary.
- In case of severe attack spray neem products. Weekly applications of simple neem products have given control of Epilachna beetles in other crops such as cucurbits.

© A. M. Varela, icipe
The maize leafhoppers (Cicadulina spp.)
The adults are about 3 mm long, slender and cream to pale yellow green in colour. These leafhoppers have two small black spots between the eyes and brown marks behind the eyes extending along the body. They have brown lines along the wings. They usually hop away when disturbed. The direct damage cause by maize leafhoppers by sucking plants is insignificant, but the indirect damage is high because they transmit the maize streak virus, a major disease of maize. Cicadulina mbila is the most important vector.
Control of the maize leafhoppers is difficult since they are very active, remain infectious for a long time and are very quick in transmitting the virus.
- Plant maize well away from grassland or previously irrigated cereals; in particular, avoid planting downwind of such areas. The numbers of leafhoppers generally increase in irrigated cereals and grasslands - or in wild grasses during rainy seasons. Leafhoppers disperse away from these areas when dry.
- Plant early - and if possible planting in an area should be carried out at the same time. Staggered planting of crops will favour multiplication of leafhoppers and increase the risk of virus transmission to later plantings.
- Keep the fields free from weeds, in particular grasses.
- Leave a barrier of 10 m of bare ground between maize fields and previously infested crops. This is reported to reduce virus incidence, by restraining movement of leafhoppers.
- Remove residues of cereal crops since they serve as infection sources.
- Use resistant varieties where available.
- They are attracted to bright green surfaces, so can be caught in sticky green traps (see picture).

© A.A. Seif, icipe

Leafhopper…

Maize leaf…

Leafhopper
The maize plant hopper (corn lantern fly) (Peregrinus maidis)
It is 4-5 m long and greyish in colour. The transparent wings are about twice as long as the body and show marked dark-brown veins. It is commonly found in groups in the funnel of the plants, the whorl, leaf sheath or underside of leaves. This insect produces large quantities of honeydew. As a result, sooty mould is often evident near the sites of aggregation. Nymphs and adults are in close association with ants, which feed on the honeydew produced by this plant hopper.
This plant hopper transmits the maize mosaic nucleorhabdovirus (MMV), maize stripe tenuivirus (MSpV), and maize line virus that can become a limiting factor in maize production.
- Practise crop rotation (alternate maize with cotton, root crops, and other non-graminae crops) to break the lifecycle of plant hoppers.
- Plough under or burn stubbles and plant debris right after harvest to kill remaining eggs, nymphs and adults.
- Avoid excessive N-fertiliser applications (N makes plant susceptible and attractive to maize plant hoppers).
- Keep planting distance wide enough for sunlight to penetrate (shady areas favour the maize plant leafhopper.
- Conserve natural enemies. Important plant hopper natural enemies are parasitic wasps (they parasitise eggs and nymphs), mirid bug (prey on eggs), dragonflies and damselflies (prey on moving adults), spiders and earwigs (prey on nymphs and adults). Intercropping with legumes is recommended as harbourage of natural enemies, as soil conditioners, and for added income.

© Courtesy EcoPort (http://www.ecoport.org) : Jannette Mitchel, ARC Pretoria
Maize streak virus
The virus causes a white to yellowish streaking on the leaves. The streaks are very narrow, more or less broken and run parallel along the leaves.
The virus is transmitted by leafhoppers (Cicadulina mbila and C. bipunctella zeae). Maize streak virus is a serious constraint to maize production in sub-Saharan Africa. The reduction in yields depends on the time of infection. Plants infected at early stage usually do not produce any cobs. Yield losses in East Africa vary between 33 and 55% under natural infection conditions. In Nigeria, 75-100% of maize plants can be infected at the end of the growing season. However, resistant varieties in these areas appear to withstand these epidemics (Anon., 1983). Sugarcane, sorghum, millet, wheat, barley, oats, rye and wild grasses can also be severely affected.
- Use of tolerant, resistant varieties if available.
- Plant early in the season.
- Eradicate grass weeds.
- Control vectors.

© A. A. Seif, icipe
Northern leaf blight Exserohilum turcicum (Helminthosporium turcicum / Dreschslera turcica / Trichometasphaeria turcica / Setosphaeria turcica)
Small oval (egg-shaped) spots first appear as water-soaked areas. They are dark and greyish-green in colour, turning greenish tan. With age they get bigger and become cigar-shaped. After rains or heavy dews, spores develop abundantly on both surfaces of the spots, particularly at the centres, giving a dark-green, velvety look to the spots. The spots may join and form large areas, which may kill entire leaves. Heavily infected leaves appear dry as if affected by drought. The disease is favoured by heavy and frequent rains, high relative humidity (above 90%) and relatively low temperatures (20-25°C). Warm dry conditions check disease development.
- Use of tolerant, resistant varieties if available
- Practise good field sanitation. Remove or plough in crop residues after harvest.
- Practise crop rotation.

© Courtesy EcoPort (http://www.ecoport.org): David C. Nowell
Purple witchweed (Striga spp.)
The parasitic weeds Striga spp. known as witchweeds, are important pests of maize, particularly in drier areas. The weeds grow on the roots of maize affecting development of maize plants. The young weeds tap the roots of maize plant and draw water and nutrients. A single weed plant produces many thousands of tiny seeds that survive in the soil for long periods. A heavy infestation can cause complete yield loss.
Striga weeds infest 40% of the arable land in the savannah region, causing annual crop losses of 7 to 13 billion dollars. Around the Lake Victoria basin infestation by Striga hermonthica causes 30 to 100% loss in maize yield. Striga infestation is associated with increased cropping intensity and declining soil fertility. Witchweed infestation has resulted in the abandonment of much arable land by farmers in Africa. The problem is more serious in areas with low soil fertility and rainfall.
None of these methods described will, alone, provide complete control and without complete control there is the certainty that surviving plants will mature and replenish the soil seed bank. Therefore, integration of one or more methods is essential for any substantial reduction of the problem. Furthermore, such integrated treatments will almost certainly need to be repeated over a number of years for long-term control.
- Weed regularly. This is the conventional method for striga control, but is time-consuming and labour-intensive.
- Rotate maize with trap crops. Some plants, such as such as sunflower, pulses and cotton, stimulate the germination of striga seeds, but also inhibit post-germination growth of the weed. Thus, although the seeds germinate, striga cannot develop successfully in these roots.
- Intercrop maize with Desmodium or other legumes. Desmodium have been shown to be more effective in reducing striga when interplanted with maize in the field than other legumes such as cowpea, soybean and sun hemp. Desmodium progressively reduces the number of striga seeds in the soil. For more information on push-pull click here
- Use resistant/tolerant varieties. Some maize varieties show partial resistance, such as "Katumani" in Kenya.

© Courtesy EcoPort (http://www.ecoport.org): C. David C. Nowell
Satintail (Imperata cylindrica)
In south-western Nigeria, satintail is a major weed reducing maize yields. The rhizomes of this weed often reduce the efficacy of farmers' weed control practice (slashing followed by 2-4 times of additional weeding) and contribute to high yield losses. In field trials grain yield was 62% less than in fields where rhizomes had been removed from soil before sowing maize.
Southern leaf blight Bipolaris maydis (Helminthosporium maydis / Cochliobolus heterostrophus)
Symptoms first appear as small yellow dots that become elongated between veins. They later become brownish to creamy white in colour with reddish to purplish brown borders. Light brown leaf spots with a brown margin, at first elliptical, becoming rectangular, up to 25 mm long and 2 - 6 mm wide. The spots are at first restricted by the leaf veins, but later they may merge. Leaves dry out and die prematurely.
Silks, portions of the husks and cobs may turn black. A black mould may develop on cobs. Disease development is promoted by prolonged wetness on foliage, extended dew, RH (97-100%) and relatively warm temperatures (24-35° C).
Spread is by airborne spores; and the fungus is also seed-borne. Survival in soil occurs for up to 12 months.
- Use disease-free seed or treated seed (steam-air mixture at 53.9 - 55°C for 17 minutes or by treatment with fungicides)
- Practise field sanitation, destroy crop residues and volunteer plants
- Practise crop rotation
- Use tolerant, resistant varieties if available

© Courtesy EcoPort (http://www.ecoport.org) : LandCare Ltd., New Zealand
Southern rust (Puccinia polysora)
Symptoms resemble those of common rust, particularly in the uredial stage (urediospores). The cinnamon-brown pustules tend to be smaller and more circular in outline than those of common rust. Pustules of telial stage (teliospores) are chocolate brown to black and circular to elongate. They are distinguished from common rust by retention of the epidermis of the leaf over the pustule for a long time. No alternate host has been reported for Southern rust.
- Use resistant varieties / hybrids, if available.
Spider mites
They can damage maize from the seedling stage to maturity. The presence of small, faint yellow blotches on the lower leaves is an indication of spider mite injury. As the colonies of mites increase in size they cause the lower leaves to become dry. The mites then migrate to the upper leaves. In Africa several species of spider mites have been reported on maize (mainly Tetranychus spp. and Olygonichus spp.). In Kenya, they are occasionally found on maize, but usually they are not of economic importance.
- Provide good growing conditions for the crop.
- Conserve natural enemies. Predatory mites and anthocorid bugs usually control spider mites.

© Image supplied by Warwick HRI, University of Warwick
Stemborers: Spotted stemborer (Chilo partellus)
Stemborers are the most important insect pests of maize in sub-Saharan Africa. Yield losses vary between 10-70%. Several species have been reported. The importance of a species varies between regions, within a country or even the same eco-region of neighbouring countries. At least four species attack maize in eastern and southern Africa, with yield losses reported to vary from 20 to 40%, depending on agro-ecological conditions, crop cultivars, agronomic practices and intensity of infestation.
The most important are the African maize stalkborer (Busseola fusca) (see above) and the spotted stemborer (Chilo partellus). The pink stalkborer (Sesamia calamistis) and the sugarcane stalkborer (Eldana saccharina) are of minor importance in maize.
Early warning signs: Young plants have pinholes in straight lines across the newest leaves. This is the time to treat - before the caterpillars move into the stem.
- Conserve natural enemies. Parasitic wasps and predatory ants are important in natural control of stemborers.
- Destroy crop residues to kill pupae left in old stems and stubble and prevent carry-over populations. This helps in limiting initial establishment of stemborers on the following season's crops.
- Intercrop maize with crops that are non-hosts for stemborers (e.g. cassava and grain legumes)
- Intercrop maize with a repellent plant such as desmodium and plant an attractive trap plant, such as Napier grass, as a border crop around this intercrop to protect maize from stemborers. This technology is known as "push-pull". For more information on push-pull click here
- Use neem products. Simple neem products are reported to be effective for control of stemborers. Place a small amount of neem powder (ground neem seeds) mixed with dry clay or sawdust at a rate of 1:1 in the funnel of the plant. One kg powder should be sufficient to treat 1500 to 2000 plants. Rainwater dissolves the active substances in neem powder as it gathers in the funnel and washes out the powder. Where rainfall is irregular a liquid neem seed extract can be sprayed into the funnel.

© Stemborer team, icipe

Spotted st…

Spotted st…

Spotted st…
Termites (Microtermes spp., Macrotermes spp., Allodontermes spp., and Odontotermes spp)
Often referred to as "white ants", they occasionally cause partial or total defoliation of maize seedlings, but are mainly damaging to older maize plants. Severely damaged plants may lodge and be completely destroyed by termites. The longer a field has been cultivated, the greater will be the yield losses caused by termites. Their feeding inside the stems causes the plant to wither and sometimes die. Termites begin to attack the roots and stems about 3 months after planting, and eventually cover them with tunnels built of soil. As plants mature the amount of damage increases rapidly. Infestation is particularly serious in dry season. It has been established that termites can damage up to 25% of maize crops in Malawi (WISARD Project Information, 2001).
- Promote conditions for healthy plant growing to prevent termite damage.
- Plough field to destroy the termites' nest, runways, and tunnels and to expose them to predators, such as ants, birds, chicken, etc.
- Practise crop rotation to reduce the build-up of termites.
- Remove plant residues and other debris especially moist and decaying woods.
- Harvest at the right time, as termites often attack maize left in the field after maturity. Attacked stalks may fall down and the termites may attack the cobs and panicles.
- Where there is risk of termite infestation, avoid leaving the crop in the field after harvest on stooks, stacks or windrows.

© A. M. Varela, icipe
White grubs
White grubs are the larvae of scarab "chafer" beetles. They are white, C-shaped with a brown head and 3 pair of legs. Some species of whitegrubs (e.g. Phyllophaga spp, Heteronychus spp.) feed on roots of maize plants. Root damage is manifested by wilting seedlings, poor stands, and patches of tilted or lodged plants showing uneven growth. Injured plants can easily be pulled out of the ground.
Feeding of adults on maize leaves is usually not of economic importance. However, adults of the black maize beetles (Heteronychus spp.) are reported as major pests of cereals in many parts of Africa. They eat the stems of young shoots just below the ground. One adult beetle may destroy several seedlings in a row.
- Remove old plants and weeds before planting.
- Plough and harrow the field to expose eggs and grubs to predators (e.g. ants and birds) and to desiccation by the sun. Once exposed, they can also be picked by hand. This is feasible in small plots.
- Provide conditions for growing healthy plants. They can tolerate grub feeding without serious damage.
- Ensure proper drainage. Grubs love moist soil, especially with decaying organic matter. Female beetles prefer to lay eggs on moist-decaying organic matter.
- Avoid planting maize immediately after old pasture in areas where grubs are frequently seen.
- Practise crop rotation. In particular, in fields where whitegrubs are common.
- Use trap crops and / or repellent plants. Good trap crops are African marigold, sunflower, and castor. Repellents plants are chives, garlic, tansy, and catnip. The crops trap and repel adult beetles from attacking the main crop grown (Golden Harvest Organics, 2003).

© A.M. Varela, icipe
| General Information and Agronomic Aspects | Information on Weeds | |||
| Information on Pests | Information Source Links | |||
| Information on Diseases |
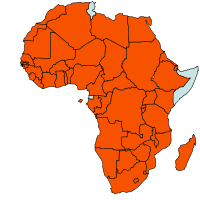 |
| Geographical Distribution of Maize in Africa |
Maize is also an important livestock feed both as silage and as crop residue, grain and is also used industrially for starch and oil extraction.
Nutritive Value per 100 g of edible Portion
| Raw or Cooked Maize | Food Energy (Calories / %Daily Value*) |
Carbohydrates (g / %DV) |
Fat (g / %DV) |
Protein (g / %DV) |
Calcium (g / %DV) |
Phosphorus (mg / %DV) |
Iron (mg / %DV) |
Potassium (mg / %DV) |
Vitamin A (I.U) |
Vitamin C (I.U) |
Vitamin B 6 (I.U) |
Vitamin B 12 (I.U) |
Thiamine (mg / %DV) |
Riboflavin (mg / %DV) |
Ash (g / %DV) |
| Maize Flour | 364 / 18% | 76.4 / 25% | 5.1 / 8% | 8.7 / 17% | 5.0 / 1% | 263 / 26% | 1.7 / 10% | 381 / 11% | - | - | 0.5 / 23% | - | 0.2 / 11% | 0.2 / 14% | 1.4 |
| Yellow Maize cooked | 108 / 5% | 25.1 / 8% | 1.3 / 2% | 3.3 / 7% | 2.0 / 0% | 103 / 10% | 0.6 / 3% | 249 / 7% | 2.0 IU / 0% | 6.2 / 10% | 0.1 / 3% | 0.0 / 0% | 0.2 / 14% | 0.1 / 4% | 0.7 |
| Maize Vegetable Oil | 884 / 44% | 0.0 / 0% | 100 / 154% | 0.0 / 0% | 0.0 / 0% | 0.0 / 0% | 0.0 / 0% | 0.0 / 0% | 0.0 IU / 0% | 0.0 / 0% | 0.0 / 0% | 0.0 / 0% | 0.0 / 0% | 0.0 / 0% | 0.0 |
Maize is especially sensitive to moisture stress around the time of tasselling and cob formation. It also needs optimum moisture conditions at the time of planting. In the tropics it does best with 600 - 900 mm of rain during the growing season. Maize can be grown on many soiltypes, but performs best on well-drained, well-aerated, deep soils containing adequate organic matter and well supplied with available nutrients. The high yield of maize is a heavy drain on soil nutrients. Maize is often used as a pioneer crop, because of the high physical and chemical demands it makes to the soil. Maize can be grown on soils with a pH from 5 - 8, but 5.5 - 7 is optimal. It belongs to the group of crops that is considered to be sensitive to salinity. Since a young crop leaves much of the ground uncovered, soil erosion and water losses can be severe and attention should be paid to adequate soil and water conservation measures.
- Local seed. Low to medium yields, usually well sheathed and so more resistant to weevil attack in storage, possibly more palatable to local tastes. Example: Kikuyu maize. Exotic varieties of maize can be collected to add genetic diversity when selectively breeding new domestic strains.
- Hybrids. High yielding but also requiring large amounts of fertiliser. Seed from hybrids cannot be saved for planting so new hybrid seed is required each year.
- Composite (e.g. "Katumani", "Coast Composite"). These are stabilised varieties and new seed is not required each year. If proper selection procedures are followed, farmers can use their seeds selected from their harvest.
| Ecozone and main areas where found | Recommended varieties | Maturity (Months) |
Yield potential (90 kg bags/acre) |
Resistance |
| Highland zones
Altitude: 1700-2100 m above sea level |
"H6213" "H 6212" "H 6210" "H 9401" "H 629" "KH 600-15A" |
6-8 6-8 6-8 6-8 6-8 6-8 |
52 52 50 48 48 40 |
|
| Highland zones with high rainfall:
Altitude: 1500-2100 m above sea level Areas: Trans Nzoia, Uasin Gishu, Nakuru, Kericho, Nandi, Bungoma, Laikipia, Kisii, Narok, and Tea zones of Central and Eastern Province West Pokot, Nyeri, Lower Nyandarua and upper Kiambu |
"H 627" "H 626" "H 625" "H 614 D" ------------ "H 6213" "H 6210" "KH 600-14E" |
5-6 5-6 5-6 5-6 ----------- 6-8 6-8 5-6 |
42 38 34 32 ----------- 52 50 34-38 |
---------------------- Rust, grey leaf spot, stem and leaf blight As above |
| Lower Highland zones, high rainfall
Altitude: 1000-1700 m above sea level Areas: Baringo, Siaya, Kisumu, Busia, Bungoma, Kakamega, Nakuru, South Nyanza, Taita Taveta |
"H 632" "H 624" "H 623" "H 516" "H 515" "H 513" "H 511" |
6-8 5-6 5-6 3.5-4.5 4-5 3.5-4.5 3.5-4.5 |
24 32 28 28 26 24 23 |
Ear rot, rust, GLS, stem and leaf blight |
| Coffee zone medium long growing
Season
Altitude: 1000-1800 m above sea level Areas: Coffee zones of Central and Eastern Provinces, Kisii, Narok, Nakuru, Siaya, Kisumu, Busia, Kakamega, Bungoma, West Pokot, Keiyo, Marakwet |
"H 513" "pH B 3253" "H 512" "H 511" "CG 4141" "CG 5222" "H 516" |
4-5 4-5 4-5 4-5 4-5 4-5 3-4 |
20 20 18 16 18 18 28 |
Ear rot, rust, GLS, stem and leaf blight |
| Dryland Areas: Marginal areas with low rainfall (400-800 mm)
Altitude: 1000-1800 m above sea level Areas: Kitui, Machachos, West Pokot, Makueni, Kajiado, Isiolo, Lower Meru and Embu, Siaya, Kisumu Altitude: 800-1200 m above sea level: Drier areas, same as for Katumani composite |
"Katumani Composite" "Makueni" "DLC1" "DH 01" "DH 02" "DH 03" "DH04" |
3-4 3-4 3-4 3-4 3-4 3-4 3-4 |
12 11 15 14 14 14 19 |
|
| Lowland Zones: Hot humid
Altitude: 1-1200 m above sea level |
"PH 4" "Pwani Hybrid 1" "Coast Composite" |
3-4 3-4 4-5 |
18 16 14 |
source: AIC 2002, KEPHIS and The Organic Farmer Feb 2007
Medium Altitude Agro-Ecozone
Transitional Zone
Lowland Agro-Ecozone
Dryland Agro-Ecozone
Some examples of maize varieties in Tanzania
- "Kilima, "UCA": suitable for medium to slightly high altitude (900-1700 m); maturity of 110-130 days; yield potential of 45-65 bags of 90 kg / ha
- "Staha": suitable for low to medium altitude (1-900 m); maturity of 110-130 days; tolerant to drought and also humid conditions
- "TMV-1": suitable for low to medium altitude (1-900 m); maturity of 110-120 days
- "Katumani, "Kito": suitable for low to medium altitude (1-750 m); maturity of 90 days; yield potential of 22-30 bags of 90 kg / ha; drought tolerant
- "Situka": suitable for medium altitude (500-1600 m); maturity of 110-120 days; yield potential of 45-65 bags of 90 kg / ha; tolerant to low nitrogen; resistant to cob rots, grey leaf spot and maize streak virus
Some examples of maize varieties in Uganda
- "Longe 4 (LP16)": maturity of 100-115 days; yield potential of 40-55 bags of 90 kg / ha; resistant to maize streak virus, northern leaf blight and grey leaf spot.
- "Longe 5 (Nalongo)"; maturity of 115 days; potential yield of 40-50 bags of 90 kg / ha; quality protein maize with lysine and tryptophan amino acids; drought tolerant; resistant to maize streak virus, grey leaf spot; susceptible to northern leaf blight.
- "Longe 8 H": suitable for mid-altitude; maturity of 120-125 days; potential yield of 88--10 bags of 90 kg / ha; excellent husk cover; tolerant to cob rots, drought and poor soil; resistant to maize streak virus, northern leaf blight and grey leaf spot; a very popular hybrid in Uganda.
For pure stand of maize in Kenya the Ministry of Agriculture recommends spacing between rows of 75 cm and between seeds 30 cm for all areas with adequate rainfall, resulting in a total plant population of 44,000. In the coffee zones this can be increased to 75 cm x 25 cm giving total plant population of 53,000 plants/ha. In dry or marginal areas the recommendation is to increase spacing to 90 cm between rows and 30 cm between seeds - total population 37,000 plants /ha. Approximate seed rate is 25kg/ha.The depth of planting is commonly 3-6 cm, depending on soil conditions and temperature. Deep sowing is recommended on light, dry soils. Animal manure or fertilisers are applied at the time of planting.
Weed control
Weed control is very important. Maize is very sensitive to weed competition during the first 4-6 weeks after emergence. It should be planted as soon as possible after the preparation of the seedbed. Inter-row cultivation to control weeds and to break up a crusted soil surface may be done until the plants reach a height of about 1 m. In Kenya 2 weedings are necessary for most maize varieties, though a third weeding may be necessary for varieties that need 6 to 8 months. Weeding by hand requires a minimum of 25 man-days/ha.
Water management
Irrigation is used in areas of low rainfall and is particularly valuable at the time of tasselling and fertilisation. Irrigation is necessary for production of green maize.
Fertilisation
Maize usually responds well to fertilisers, provided other growth factors are adequate. The quantity of manure applied by smallholders is usually very limited. Improved varieties can only reach their high yield potential when supplied with sufficient nutrients. A maize crop of 2 t/ha grains and five t/ha stover removes about 60 kg N, 10 kg P2O5 and 70 kg K2O from the soil. Nitrogen uptake is slow during the first month after planting, but increases to a maximum during ear formation and tasselling. Maize has a high demand for nitrogen, which is often the limiting nutrient. High nitrogen levels should be applied in three doses, the first at planting, the second when the crop is about 50 cm tall, and the third at silking.
Many soils provide substantial amounts of the phosphorus (P2O5) and potassium (K2O) but this is not adequate enough, especially at the seedling stage. Apply P2O5 near the seed for early seedling vigour. K2O is taken up in large quantities but plants' requirement can usually be estimated by soil analysis. K2O deficiency results in leaves with burnt edges and yellow or light green colour and empty cob ends, while P2O5 deficiency results in purple tinged leaves and hollow grains. Nitrogen deficiency shows as yellow or light green stunted plants.
Phosphate is not taken up easily by maize and, moreover, some tropical soils are deficient in available phosphate. Zinc deficiency symptoms include shortening of internodes and light streaking of leaves followed by a broad stripe of bleached tissue on each side of the leaf midrib. Occasionally the leaf edges and interior of the stalk at the nodes appear purplish. It is advisable to apply organic manures to improve soil structure and supply nutrients, all before ploughing.
Nitrogen (N) can be applied in organic farming via green manure (legumes fixing N directly from the atmosphere), farmyard manure (FYM) or compost. Phosphorus can be supplied through FYM, compost, and in the form of rock phosphate (available in East Africa as Mijingu rock phosphate). rock phosphate should be applied in the rows or planting holes at planting to promote root formation., Potassium can be supplied through FYM, compost and ashes.
However, fertiliser recommendations based on soil analysis provide the very best chance of getting the right amount of fertiliser without over or under fertilising. Ask for assistance from a local agriculturist office.
In rain-fed maize growing areas, plant seeds along with the first rain. This will allow roots to absorb the natural nitrates formed with bacterial action in the soil. Roots are susceptible to poor drainage, which causes stunted and yellowing of leaves. Stagnant water results to loss in N through leaching and denitrification (FADINAP, 2000).
For more information on organic plant nutrition click here.
Intercropping
In Africa maize does well when intercropped with beans or other legumes. The intercropped legumes should be sown at the time of first weeding in order not to crowd out the young maize plants. Since maize is a heavy feeder and takes considerable nutrients out of the soil, it can only be grown continuously on the richest soils or when heavily fertilised. Recommended legumes for intercropping in Kenya are beans, pigeon peas, cowpeas, groundnuts and soybeans. Other crops that have been tried with varying success include potatoes, cassava and pumpkin.
Intercropping maize with beans and other legumes regulates pests (leafhopper, leaf beetles, stalk borer, and fall armyworm) and increases the land utility. Intercropping Canavalia (Canavalia spp.) with maize improves soil productivity. Sow Canavalia seeds 4 weeks after sowing maize. Place 1 seed/per hole in a row between maize rows with 50 cm between holes. Allow Canavalia to grow after harvesting maize until it is time to plant the next crop. Then plough the plant materials into the soil (CIAT, 2000).
Intercropping maize with beans and squash enhances parasitism of caterpillars. This practice increases food sources for beneficial insects whereby increasing abundance of natural enemies. The intercropping system of maize-beans-squash is a low input and high yield strategy in the tropics. Maize yield is increased by as much as 50% over monoculture yield. Although the yields for beans and squash are reduced, the overall yield for the 3 combined crops is greater than when grown separately in monocultures (Agroecology Research Group, 1996).
Push-pull
Desmodium (Desmodium uncinatum) and molasses grass (Melinis minutifolia) when planted in between maize rows keep the stem borer moths away. These plants produce chemicals that repel stem borer moths. In addition desmodium supresses the parasitic witchweed Striga hermonthica. Napier grass (Pennisetum purpureum) and Sudan grass (Sorghum vulgare sudanese) are good trap crops for stem borers. Napier grass has its own defence mechanism against crop borers by producing a gum-like-substance inside its stem, this prevents larva from feeding and causing damage to the plant. Both grasses attract stemborer predators such as ants, earwigs, and spiders. Sudan grass also increases the efficiency of natural enemies, in particular parasitic wasps, when planted as border crops (Herren; Pickett, 2000; ICIPE, 2006). For more information on push-pull click here
Alternative uses of maize in mixed cropping
- Shading of vegetable crops by planting single rows between vegetables in areas of high intensity of sunshine can increase yields of intercropped vegetables.
- Use as support for runner beans for export or local consumption.
Maize for home consumption is either sun-dried on the cob for several days by hanging up tied husks, or put in a well-ventilated store or crib. Easy test for moisture content: take a few grains and try to crush them with your teeth - below 13% moisture level the grains are extremely hard and almost impossible to crush this way. Shelling (the removal of grains from the cob) is usually carried out by hand, though several hand and pedal-powered mechanical shellers are now available. The average recovery is about 75%. The shelled grain is dried again for a few days and then stored in bags, tins or baskets.
The optimum moisture content for storage is 12-13%. In Indonesia seed for the next crop is generally selected from the last harvest. The selected cobs are stored at home in the husk above the fireplace to prevent losses by insects. Crop residues are removed from the field and then used as fodder, fuel, etc.
Stemborers and striga weed account for losses in maize in the eastern and southern Africa region of 15-40% and 20-100%, respectively. When they occur together, farmers can lose their entire crop (ICIPE, 2006). Earworms and armyworms are other major pests.
The principal pests of stored maize are Angoumois grain moth (Sitotroga cerealella), the larger grain borer (Prostephanus truncatus), maize weevils and rodents.
|
Grasshoppers and locusts Several species of grasshoppers and locust feed on maize. The edible, a long horned grasshopper Homorocoryphus nitidulus vicinus (Ruspolia differens) has been reported to occasionally attack maize in Tanzania (Bohlen, 1973). This grasshopper attacks maize in the silking stage, arresting pollination. Other grasshoppers and locust attack maize from the mid-whorl stage to maturity, and may consume every part of the plants. Attacks vary in severity from location to location. | 
Grasshopper
Grasshopper Ruspolia nitidula in Uganda © Kurt Kulac |
|
What to do:
|
|
Stemborers: African maize stalkborer (Busseola fusca) Stemborers are the most important insect pests of maize in sub-Saharan Africa. Yield losses vary between 10-70%. Several species have been reported. The importance of a species varies between regions, within a country or even the same eco-region of neighbouring countries. At least four species attack maize in eastern and southern Africa, with yield losses reported to vary from 20 to 40%, depending on agroecological conditions, crop cultivars, agronomic practices and intensity of infestation.
| 
African maize stalkborer
African maize stalkborer (Busseola fusca) damage on maize. Caterpillars are relatively featureless and noctuid, growing to a length of up to 4 cm. They lack conspicuous hairs or markings and look smooth and shiny. Colour is variable but usually creamy-white © A. M. Varela, icipe |
|
What to do:
|
|
Stemborers: Spotted stemborer (Chilo partellus) Stemborers are the most important insect pests of maize in sub-Saharan Africa. Yield losses vary between 10-70%. Several species have been reported. The importance of a species varies between regions, within a country or even the same eco-region of neighbouring countries. At least four species attack maize in eastern and southern Africa, with yield losses reported to vary from 20 to 40%, depending on agro-ecological conditions, crop cultivars, agronomic practices and intensity of infestation. | 
Spotted stemborer
Caterpillar of the spotted stemborer (Chilo partellus) is about 2.5cm long © Stemborer team, icipe |
|
What to do:
|
|
African bollworm (Helicoverpa armigera) Caterpillars of the African bollworm also known as the corn worm or earworm attack mainly the developing cobs, although they may occasionally feed in the leaf whorl or on tender tassels. Eggs are laid on the silks. Caterpillars invade the cobs and feed on developing grain. Development of secondary infections is common. Local outbreaks of this pest are sometimes severe. | 
African bollworm
African bollworm on beans - Caterpillars are 3-4 cm in length. © A.M. Varela, icipe |
|
What to do:
|
|
The African armyworm (Spodoptera exempta) The African armyworm is a very damaging pest, capable of destroying entire crops in a matter of weeks. Although they are regarded as occasional pests, in an outbreak large number of caterpillars will appear destroying the whole plant to ground level. | 
African armyworm
African armyworm (Spodotera exempta). Mature caterpillars measure up to 4 cm. © University of Arkansas |
|
What to do:
|
|
Purple witchweed (Striga spp.) The parasitic weeds Striga spp. known as witchweeds, are important pests of maize, particularly in drier areas. The weeds grow on the roots of maize affecting development of maize plants. The young weeds tap the roots of maize plant and draw water and nutrients. A single weed plant produces many thousands of tiny seeds that survive in the soil for long periods. A heavy infestation can cause complete yield loss. | 
Striga
Striga (Striga hermonthica) weeds in maize field. © Courtesy EcoPort (http://www.ecoport.org): C. David C. Nowell |
|
What to do:
|
- AIC, Kenya (2002). Field Crops Technical Handbook.
- Agroecology Research Group. Corn-bean-squash intercrop in Mexico. www.agroecology.org
- Asean IPM Knowledge Network Management. Management of corn plant hoppers in the Philippines.
- Borgemeister, C., Holst, N., Hodges, R. J. (2003). Biological control and other pest management options fo larger grain borer Prostephanus truncatus. In Biological Control in IPM Systems in Africa. Neuenschwander, P., Borgemeister, C and Langewald. J. (Editors). CABI Publishing in association with the ACP-EU Technical Centre for Agricultural and Rural Cooperation (CTA) and the Swiss Agency for Development and Cooperation (SDC). pp. 311-328. ISBN: 0-85199-639-6.
- Brunt, A.A., Crabtree, K., Dallwitz, M.J., Gibbs, A.J., Watson, L. and Zurcher, E.J. (eds.) (1996 onwards). `Plant Viruses Online: Descriptions and Lists from the VIDE Database. Version: 20th August 1996.' URL http://biology.anu.edu.au/Groups/MES/vide/
- CAB International (2005). Crop Protection Compendium, 2005 Edition. Wallingford, UK www.cabi.org
- FADINAP. Integrated plant nutrition systems. www.eldis.org
- Herren, H., Pickett, J. (2000). Kenya: Vuta-sukuma (Push-pull) pest management in smallholder systems. ICIPE annual reports.
- ICIPE (2003). Development of biocontrol-based management of Helicoverpa armigera in eastern and southern Africa. 2000-2003 ICIPE Scientific Report. International Center for Insect Physiology and Entomology, Nairobi, Kenya. www.push-pull.net
- ICIPE. Implementation of habitat management strategies for the control of the stemborers and striga in maize-based farming systems in Eastern Africa and mechanisms of striga suppression by Desmodium sp. www.push-pull.net
- IITA. www.iita.org
- Kranz, J., Schumutterer, H., Koch, W. (1977). Diseases, pests and weeds in tropical crops. Verlag Paul Parey. ISBN: 3-489-68626-8.
- Le Pelley, R. H. (1959). Agricultural insects of East Africa. East African High Commission. Nairobi, Kenya.
- Ministry of Agriculture 2006: Economic Review of Agriculture
- Nutrition Data www.nutritiondata.com.
- OISAT: Organisation for Non-Chemical Pest Management in the Tropics www.oisat.org
- Ortega, A. O. (1987). Insect pests of maize. A guide for field identification. Mexico, D. F.: CIMMYT. ISBN 968-6127-07-0
- Songa J.M., Overholt W.A., Mueke J.M., Okello R.O., (2002). Farmers' perceptions of aspects of maize production systems and pests in semi-arid eastern Kenya: factors influencing occurrence and control of stem borers. International Journal of Pest Management, 48 (1):1-11.
- Terry, P. J. and Michieka, R. W. (1987). Common weeds of Africa. Food and Agriculture Organization of the United Nations (FAO). ISBN 92-5-002426-6.
- CIMMYT Maize Program (2004). Maize diseases: A guide for field identification. 4th edition. Mexico, D. F: CIMMYT. ISBN 970-648-109-5
- The Organic Farmer Feb 2007
- WISARD PROJECT INFORMATION (2001). Biology and management of termites and white grubs in smallholder cropping systems.
- Youdeowei, A. (2002). Integrated pest management practices for the production of cereal and pulses. Ministry of Agriculture (MOFA) Plant Protection and Regulatory Services Directorate (PPRSD), Ghana, and the German Development Cooperation (GTZ). ISBN: 9988-0-1086-9.
 Back
Back
