
|
Cotton
Scientific name:
Gossypium spp.
Order/Family:
Malvales: Malvaceae
Local names:
Pamba (Swahili)
Pests and Diseases:
African bollworm
African cotton mosaic disease
Anthracnose
Aphids
Ascochyta blight
Bacterial blight
Cotton leaf curl bigeminivirus
Cotton leaf roller
Cotton stainers
Cutworms
Damping-off diseases
Fusarium wilt
Helopeltis bugs
Leafhoppers or jassids
Other bugs
Root-knot nematodes
Sedges
Spider mites
Whiteflies
Cotton semi-looper
|
Bollworms (Helicoverpa armigera, Earias spp and Pectinophora gossypiella)
Bollworms are among the most serious cotton pests. They feed in the bolls, damaging lint and seed and causing considerable reduction in yield and quality. The main bollworms are the African bollworm (Helicoverpa armigera), spiny bollworm (Earias spp) and the pink bollworm (Pectinophora gossypiella). Caterpillars of the false codling moth (Cryptophlebia leucotreta) are sometimes found boring in the bolls.
The African bollworms (Helicoverpa armigera) bores into the boll often with the hind part of the body exposed outside the boll. If younger bolls are attacked they normally show a yellowish colour and the bracteoles open out (flared square). One caterpillar can damage a number of bolls and buds by moving from one to the other. Infested bolls and buds drop prematurely.
2) Spiny bollworms (Earias biplaga / Earias insulana)
The adults are moths, about 12 mm long with a wingspan of about 20-22 mm. The forewings are white, peach, metallic green to straw yellow in colour according to the species. Fully-grown caterpillars are up to 18 mm long. Their body bears numerous fleshy spines. Caterpillars vary in colour from brown and deep orange to grey and yellow. Caterpillars bore into flower buds, young shoots and maturing bolls. Hollowed tender shoots whither and die.
3) The pink bollworm (Pectinophora gossypiella)
The moths are small (about 8-9 mm long) with grey brown or brown forewings covered with dark spots. The hind wings are whitish, broader than the fore wings, and have a long fringe on the posterior margin. The pink caterpillars are up to 15 mm long and have 2 broken transverse red bands on each segment of the body. This is usually a late season pest.
The caterpillars bore into the flower buds and young bolls causing shedding. Caterpillars feeding on flowers spin the petals together, causing the formation of what is called "rosette flowers" which do not open up. The bolls open prematurely and may also rot or drop to the ground. The most important damage is caused by caterpillars penetrating bolls, where they feed on the seeds and soil the lint with frass and excrements. The pest is carried over to the next season and crop as a diapause-caterpillar mainly within the cottonseeds.
- Practise field hygiene. Remove and destroy old crops and plant debris after harvesting or let cattle graze in the field after the picking is over.
- Crop rotation with plants not related to cotton (avoid kenaf, okra, abutilon and other malvaceas) may help to reduce attack by the pink bollworm and the spiny bollworm, but it is unlikely to be effective against the African bollworm, since this pest feed on many different plants.
- Mixed cropping helps to reduce attack by bollworms; plant composition and combinations are important to optimise the benefits. Some plants may act as trap crops and/or may attract natural enemies that will then predate on bollworms.
- In Tanzania, early sowing of "Ukiriguru" varieties is strongly recommended. These varieties have the ability to compensate for early crop loss of fruiting points caused by either physiological stress or by the African bollworm, provided soil moisture and nutrients are not limiting. Thus, the early sown cotton (sown between the end of November and end of December) may lose its bottom crop, but can compensate later by producing a crop during the main rains in March-April. If bollworms attack later, then the early sown crop would have set its main crop and will therefore escape damage (Nyambo et al., 2003).
- Encourage natural enemies like ladybird beetles, lacewings, spiders etc.
- Direct control measures are spraying with neem spray or a garlic-chilli-onion-repellent and Bt. For more information on neem click here. For information on Bt click here
- In Malawi and Zimbabwe, thresholds based on egg numbers have been used successfully in cotton since 1961. Spraying was recommended at an average of one egg per two plants in twice-weekly counts. In the Sudan Gezira, over two eggs or caterpillar per 18 plants, and in Australia two eggs per metre of row were used as thresholds (CABI, 2000). It has been argued that control thresholds based on damage are easier to use and more economical than those based on pest density. In the case of cotton, damaged buds are easier to detect and sample than either eggs or small caterpillars. Studies in Tanzania indicate that spraying at damage threshold of 10 to 20% would give adequate protection to the crop. Further fine-tuning of damage thresholds should be concentrated during the first four weeks of flowering when most of the damage by this pest occurs. (Kabissa, 1989).
- Bollworms can also be removed by hand picking. This helps when their numbers are low and in small fields.

© A. M. Varela, icipe

African bo…

Spiny boll…

Pink bollw…
African cotton mosaic disease
The disease causative agent is still unknown but it is associated with whitefly-transmitted geminiviruses, based on symptomatology typical of begomoviruses and the demonstration of its transmissibility by the whitefly Bemisia tabaci. The disease is widespread in Africa south of the Sahara. Disease symptoms include irregular leaf mottling (chlorotic patches), reduced flower production, boll shedding and stunting of infected plants.
- As with most insect-vectored plant viruses, the disease pressure may be reduced by routine sanitation practices.
- Also remove infected residues; this helps to reduce disease inoculum (source) and assists in lowering vector populations
- Remove seasonal weeds adjacent to cotton fields and ditch banks in irrigated areas

© A. Seif, icipe
Anthracnose (Glomerella gossypii)
It is caused by the fungus Glomerella (Colletotrichum gossypii). It is the primary cause of seedling blight, boll rot and fibre deterioration (staining) in all humid cotton growing areas. Seedlings affected by blight are killed before or after they emerge (damping-off).
Symptoms of seedling blight on cotyledons are usually diseased areas on margins or small reddish or light coloured spots. Diseased seedlings show reddish-brown lesions below ground. The lesions may be on one side of the stem or they may surround it and extend down the root. On the bolls the disease appears as small, round, water-soaked spots, which enlarge, become sunken and brownish in colour. In wet weather, the spots are covered with a sticky mass of fungal spores pinkish in colour.
Boll infection is often associated with wounds made by boll weevils. The lint and seeds are rapidly invaded once the disease gets through the husk of the boll. Affected lint is stained.
The disease survives on old rotten bolls, crop refuse in the field and on the seeds. It is spread on diseased seeds. Infection is favoured by moderate temperatures and high moisture.
- Use disease-free seeds.
- Practise crop rotation.
- Practise good field hygiene.

© Jürgen Kranz (Courtesy of EcoPort, www.ecoport.org)
The cotton aphid (Aphis gossypii)
Cotton aphids are about 1-3 mm long, and variable in colour from yellow to yellowish green or very dark (almost black) with two cornicles (projections) on the rear end and long antennae. They suck sap preferably from tender shoots and the underside of young leaves. Feeding by aphids may result in crinkling and cupping of leaves, defoliation, square and boll shedding and stunted growth.
Honeydew (a sugary liquid) excreted by aphids accumulates on the upper surface of leaves and sooty mould develops. Honeydew can contaminate the fibre if the bolls are open, causing problems in processing the lint.
- The cotton aphid is attacked by a range of natural enemies. The most important are ladybird beetles, hoverflies, lacewings and parasitic wasps. They usually keep aphids under control.
- Healthy cotton plants can tolerate a fairly high number of aphids.
- Avoid plant stress by giving neither too little nor too much manure. Avoid water stress and water logging.
- Intercrop cotton with maize or sorghum to create a natural balance of pests and natural enemies.
- Use yellow water or sticky traps.
- Spraying maybe necessary in the case of high aphid infestation or if the honeydew affects the lint in open bolls. If this is the case, use plant extracts such as neem leaf and seed extracts, ginger rhizome extract, and custard apple leaf extract for control of aphids.
- 3% potassium soap in acute cases; in extreme cases use nicotine extract, neem, chilli, garlic, or Lantana camara

© Ronald Smith (Courtesy of EcoPort, www.ecoport.org)
Ascochyta blight (Ascochyta gossypii)
The disease is caused by fungus Ascochyta gossypii. It may manifest as seedling blight, a leaf spot, a stem canker, and as a boll spot. The first symptoms are small, round, white, purple-ringed spots on the cotyledons and lower leaves. The spots become somewhat elongated and raised on the upper surface. Later they change to a light brown, the purple ring around the outside disappears, and the diseased tissue often falls out. The upper small leaves, petioles and buds are often infected, and the plant dies. The almost bare stems, with a few small leaves at the tip, are characteristic of the disease at the later growth stages.
Stem infection, which occurs only during consecutive days of cloudy, wet weather, leads to the formation of lesions, which may reach several centimetres in length, with cracks and ragged edges. The centre of these lesions becomes pale, liver-coloured and covered with tiny black dots (bodies of fungal spores, conidiomata). Cankers may encircle the stem and kill the distal parts. Flowers are not attacked, but mature lint can be destroyed. Lint may show a grey discolouration with conidiomata in half-opened bolls.
The disease is favoured by long periods of rain and cool weather. It is also seed-borne.
- Use disease-free seeds.
- Practise crop rotation with crops non-related to cotton (e.g. cereals).
- Practise good field sanitation and cultivation practices which destroy drop residues.

© A.M. Varela, icipe
Bacterial blight (Xanthomonas axonopodis pv. vignicola)
This disease appears as tan to brown angular leaf spots with yellow margins on leaves, pods, and stems. It may cause severe defoliation during periods of high humidity. It is seed-borne.
- Use certified disease-free seeds.
- Avoid working in the fields when it is wet.
- Practise good field sanitation.

© A.M. Varela & A.A. Seif, icipe
Cotton leaf curl bigeminivirus
Leaves of infected cotton curl upward or downward often accompanied by foliar discoloration and mosaic. These leaves may bear leaf-like enations (growth) on the underside along with vein thickening. Plants infected early in the season are stunted and yield is reduced drastically. The virus is transmitted by whiteflies (Bemisia tabaci).
- Plant resistant varieties (e.g. "B6L"; "L1530"; "XL1"; "X1730A")
- Eliminate weeds in and near cotton fields. This may have some advantage in reducing virus and insect vector reservoirs
The cotton leaf roller (Haritalodes (Sylepta) derogata)
The moths are about 15 mm long with a wingspan of 25-30 mm. They are yellowish-white with black and brown spots on the head and thorax and a series of dark-brown wavy lines on the wings. Caterpillars are dirty pale green and semi-translucent and up to 30 mm long when fully grown. Moths lay eggs on the underside of leaves. Young caterpillars feed initially on the underside of leaves, but older caterpillars spin or roll leaves together, and eat the leaf margins, causing the leaves to curl and droop. They pupate in the leaf roll or in debris on the ground.
The leaf roller is a common pest, which may cause considerable local damage. In extreme cases, the cotton plants may be almost completely defoliated, as a result the growth of the plants is stunted, and the bolls ripe prematurely leading to yield reduction.
- The leaf roller is usually controlled by natural enemies, particularly parasitic wasps, spiders and praying mantis.
- Removal and destruction of eggs, caterpillars, pupae and rolled leaves help to reduce damage.

© A. M. Varela, icipe.
Cotton stainers (Dysdercus spp.)
Several types of bugs attack cotton. They are late season pests and they usually attack during the flowering and fruiting stages of the crop. The most important are the cotton stainers.
Stainer bugs are between 14 and 24 mm long. They are bright red, yellow or light grey with an orange tinge depending on the species, and with black bands. Stainer bugs are late season pests. They appear when the bolls are ripening. Female lays whitish yellow eggs in moist soil or in crevices in the ground. They hatch to produce reddish-orange nymphs. Initially the nymphs are wingless, but wings develop gradually as the nymphs grow.
The nymphs are found together in the area where the eggs have been laid and later disperse to look for food. Both nymphs and adults feed on the bolls, but adults cause the most serious damage. They pierce through the boll and suck the seeds reducing germination capability and the quality of the seed oil and the cake.
Furthermore, they cause severe indirect damage by transmission of a fungus (Nematospora sp.), which leads to internal boll rot and stain of the lint with typical yellow colour, hence the name 'cotton stainers'. The nymphs feed mainly on seeds in open bolls reducing the seeds's oil content and their germination capacity. Severe bug attack affects yield, oil content and the marketability of the crop.
- Cotton stainers are attacked by a range of natural enemies. The most important are assassin bugs, ants, spiders, birds and parasitic flies.
- Caging chickens in cotton plots using chicken wire may control cotton stainers; about 15 birds will keep about 0.1 ha free of stainer bugs. This is a good option for small plots grown next to the homestead.
- Preventive control measures are sanitation; remove cotton plants and all its debris as well as ratoon cotton as soon as harvesting is over. Keep stores clean. Cotton should be grown strictly as an annual with a close (dead) season. Hand pick and destroy the bugs, this is feasible in small plots and at the beginning of infestations, and will help to reduce population density.
- Custard apple leaf extract is recommended for control of these bugs (PAN).
- The baobab tree is one of the main host plants of stainer bugs. If cotton is grown where baobab occurs, the soil and trunk of the baobab tree should be sprayed to kill the nymphs hatching from eggs laid around the stem.
- Tanzania: pyrethrum formulation with black wattle extract as UV light stabiliser is used.

© A.M. Varela, icipe
Cutworms (Agrotis segetum and the cotton leafworm, Spodoptera littoralis)
Caterpillars cut seedlings at ground level. They can be found in the soil up to a depth of about 5cm around the plant host. Caterpillars of the cotton leafworm are up to 45 mm long. Their colour varies from yellowish white to bluish grey and greyish brown. On the dorsal side of the caterpillars there are usually w rows of triangular black spots. These spots can be absent with the exception of those at the fore and hind part of the body. Although the caterpillars feed mainly on leaves, they occasionally cut plants and attack fruits.
- Remove weeds in and around the fields as a preventive measure to reduce the number of sites where the moths can lay eggs. Do this at least 2-3 weeks before planting.
- Plough and harrow fields properly before planting to destroy eggs and expose caterpillars to birds, ants and other predators.
- Apply neem cake or de-oiled castor cake before sowing.
- Encourage the presence of birds with trees and hedges. Also promote natural enemies like spiders, ground beetles and lacewings.
- Interplant with onion, garlic, peppermint or coriander, this will act as a repellent to cutworms.
- Sunflowers can be planted as a trap crop.

© A.M. Varela, icipe
Damping-off diseases
Cotton seedlings are subject to attack by several fungal and bacterial diseases. These include the anthracnose fungus (C. gossypii), Ascochyta blight (A. gossypii), wilt fungus (F. o. f.sp. vasinfectum), bacterial blight (X. a. pv. malvacearum), Pythium spp. and Rhizoctonia solani.
Pythium spp. are particularly serious where soil temperatures are low and wet weather prevails at planting.
Rhizoctonia solani causes sore shin disease of cotton seedlings. Attacked seedlings develop dark to reddish-brown cankers on the stems near the soil line. The cankers encircle the stems or penetrate so deeply that the plants fall over and die.
- Use disease-free seeds.
- Avoid planting during cold, wet weather.

© A.A. Seif & A.M. Varela, icipe

Damping-of…

Damping-of…
Bacterial blight (Xanthomonas axonopodis pv. malvacearum)
The disease attacks seedlings, leaves, stems and bolls. Seedling attack, usually called seedling blight, results in small, round, water-soaked spots on the cotyledons as they emerge from the seed coat. The spots furnish inoculum (disease source) for the developing true leaves. Leaf spots are translucent, water-soaked, angular and bordered by veins. Affected leaves become browned and blackened. Spread of the bacteria along leaf veins is commonly called vein blight. Affected veins appear black. Younger leaves are more susceptible to vein blight than mature, old leaves.
In older plants black lesions may develop on the stems. These lesions girdle the stem and may cause breaking of the stem when windy. This symptom is known as black arm. Infected bolls have round, shiny lesions, which later become sunken, browned and finally blackened. Infected bolls become deformed and open prematurely. Fibre in diseased bolls is usually stained.
The disease is carried over season to season on infected crop debris and diseased seeds. Spread of the disease is through use of infected seeds and in the field by wind-blown rain water.
- Plant resistant varieties (e.g. Albar 51; Albar G501; BPA; BP 52; SATU; S 2950)
- Use disease-free seeds
- Practise crop rotation of at least 3 years with cereals or legumes
- Practise good field sanitation

© A.M. Varela & A.A. Seif, icipe
Helopeltis bugs (Helopeltis schoutedeni and H. anacardii)
Other bugs feeding on cotton include Helopeltis bugs, blue bugs and cotton lygus and the cotton seed bug.
Cotton Helopeltis (Helopeltis schoutedeni)
Helopeltis bugs are up to 10 mm long and have very long antennae. They are bright red (females) or yellowish-red (males) in colour. The bugs prefer to feed on young plant tissue of leaves, shoots, peduncles and petioles. The toxic saliva injected during sucking causes pale brown necrotic patches. The leaves and the stems of the plants are twisted. The lesions on the leaves often drop out leaving holes as if attacked by chewing insects. The green boll wall is also attacked showing dark, circular, sunken lesion.
- Bugs, in particular adults are difficult to control since they can readily move from neighbouring crops or wild plants into the cotton crop.
- Do not interplant cotton with crops that are host for Helopeltis bugs, such as cashew, tea, sweet potato, guava and mango.
- Monitor the crop regularly. Helopeltis attack occurs very suddenly and great vigilance is very important to control this pest, particularly during the rainy season or when water is available leading to flushing (production of young shoots) when Helopeltis populations normally build up.
- Natural enemies are important in the control of bugs. Conserve natural enemies. Weaver ants build nests on cashew trees providing good protection against this and other bug pests. The most important are parasitic wasps attacking bug eggs; ants, which feed on eggs and nymphs; and various predacious bugs, spiders, birds and parasitic flies.
- Neem products reportedly reduce feeding by some bugs.
- Since bugs are late season pests early sowing and picking are recommended to reduce bug attack.

© F. Haas, icipe

Helopeltis…

Helopeltis…
Leafhoppers or Jassids (Empoasca spp)
Adult leafhoppers are (2 to 3 mm long) and thin. The wings are held roof like over the abdomen. They are pale green to yellowish green in colour, shiny and more or less transparent. The legs are slender with bristles. The nymphs resemble the adults but are smaller and do not have fully developed wings. Adults and nymphs suck sap from the leaves, remaining on the underside during the day, but also moving to the upper surface during the evening. When disturbed they run sideways rapidly to reach a shady part of the host plant.
Feeding by leafhoppers causes discolouration, and leaf curl, the outer zone of the leaf turns yellow to reddish and whiter later. Heavy leafhopper infestation may retard plant growth and may cause severe yield losses. Cotton fields attacked by leafhoppers are easily recognisable from some distance, owing to their reddish or purple colour ("hopperburn").
- Use resistant varieties. A number of very hairy varieties have been bred, which are considerable less prone to leafhopper attack than those varieties whose leaves are not or only sparingly covered with hairs. By planting such resistant varieties damage by leafhoppers can be avoided to large extent. In Tanzania, the release of the "Ukiriguru" varieties resistant to leafhoppers and to bacterial blight played a major role in the increase in annual crop production.
- Early sowing helps if the cotton plants have past the most susceptible plant stage during the period after the rainy season, when leafhopper population is at its peak.
- Use repellent plant extracts from: neem, chilli, garlic or Lantana camara

© Steve L. Brown, University of Georgia, Bugwood.org
Bugs (Blue bug, cotton lygus, cotton seed bug)
Other bugs feeding on cotton include blue bugs and cotton lygus and the cotton seed bug.
Blue bugs (Calidea degrii, Calidea bohemani)
The adults are large bugs, 8 to 17 mm long by 4-8 mm broad, and strikingly coloured, with red or orange underneath and the upper surface bright metallic blue or green with black spots. They suck the seeds of unopened bolls causing staining of the cotton lint in a similar way as stainer bugs; as a result the development ceases and the boll aborts.
Blue bugs seldom breed on cotton. They usually invade cotton when the bolls are well formed, from other host plants, for instance sorghum, sunflower, castor and many wild hosts such as crotalaria and hibiscus.
Cotton lygus (Taylorilygus vosseleri)
The adult bugs are up to 5 mm long and pale green to light brown in colour. The nymphs are green. Adults and nymphs prefer to suck young leaf buds, flower buds and bolls. The toxic saliva causes the flower buds to turn yellow and drop off. Leaves, which have been attacked in the bud stage, later show a ragged appearance with many irregular holes. The cotton lygus is an early season pest.
Cotton seed bug (Oxycarenus hyalinipennis)
These bugs are small (4 to 6 mm) and blackish in colour. Their wings are transparent. They attack open or damaged pods mainly at the end of the growing season. It is a late season pest, which mainly appears when the bolls have opened. Nymphs and adults suck the seeds. Feeding by large numbers of bugs reduces considerably the germination rate, seed weight and oil quality. The lint is not affected.
- Bugs, in particular adults are difficult to control since they can readily move from neighbouring crops or wild plants into the cotton crop.
- Natural enemies are important in the control of bugs; the most important are parasitic wasps attacking bug eggs; ants, which feed on eggs and nymphs; and various predacious bugs, spiders, birds and parasitic flies.
- If heavy outbreaks of the cottonseed bug occur, the cotton should be picked as soon as the cotton bolls mature.
- Neem products reportedly reduce feeding by some bugs.
- Since bugs are late season pests early sowing and picking is recommended to reduce bug attack.
- Tanzania: pyrethrum formulation with black wattle extract as UV light stabiliser

© A. M. Varela, icipe
Root-knot nematodes (Meloidogyne spp.)
Several species of root-knot nematodes attack cotton roots. Infested plants are often stunted and leaves yellow. Galls or knots are formed on the roots. Infestation can be particularly serious in light soils. Nematode infestation predisposes plants to Fusarium wilt infection.
- Practice crop rotation with crops not related to cotton (e.g. cereals) whereby cotton is cropped once every 3 or more years.

© A. M. Varela, icipe
Spider mites (Tetranychus spp; cotton red mite Oligonychus gossypii)
Spider mites feed on the lower surface of leaves. Adults are about 0.6 mm long. As a result the leaf surface appears tan or yellow and the upper surface has a speckle or mottled appearance. A close inspection of the lower leaf surface shows the mites as tiny moving greenish or reddish speckles. Heavily infested leaves turn yellow, curl up, dry and are shed. Mites produce a large amount of webbing. In heavy infestations a fine web may cover the leaves. Plants may die when infestation is severe, particularly in hot dry conditions.
- Provide good plant growing conditions, in particular enough water; water stressed plants are prone to mite damage.
- Avoid the use of broad-spectrum pesticides, which kill natural enemies and may result in mite outbreaks.
- Avoid planting next to infested fields.
- Sulphur preparations control mites. However, it should be noted that sulphur also kills predatory mites.

© O.P. Sharma, NCIPM, New Delhi. India, Bugwood.org
Whiteflies (Bemisia tabaci)
Whiteflies (Bemisia tabaci) suck the sap of leaves. Feeding weakens plants and may lead to yellowing and wilting, premature leaf shed and reduced plant growth, affecting the quality and quantity of yield. Severe attack may kill young plants. Honeydew excreted by whiteflies and sooty mould that develops subsequently can affect the fibre quality considerably and cause problems in processing the lint.
The whitefly is a vector of important virus diseases on various crops including cotton. It transmits the cotton leaf curl virus and the African cotton mosaic disease. Attacks are common during the dry season, and usually recede with the onset of rains.
- Whiteflies are attacked by parasitic wasps and predators. Conservation of these natural enemies is important.
- Yellow sticky traps are useful for monitoring whiteflies, and may help to control low populations.
- Use 3% potassium soap in acute cases
- Plant trap crops: e.g. Lablab niger

© B. Nyambo, A. A. Seif, icipe
| General Information and Agronomic Aspects | Information on Diseases | |||
| Biological methods of plant protection | Information Source Links | |||
| Information on Pests |
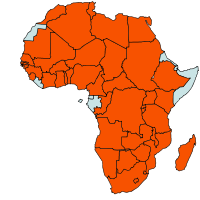 |
| Geographical Distribution of Cotton in Africa |
G. hirsutum:
- Upland cotton
- 80-90% of world market
- short to medium fibres (2-3 cm; middle stapled variety)
G. barbadense:
- Sea Island cotton
- 10-20% of global market
- high-quality, long to very long fibres (3-4 cm; long stapled variety)
In addition, the annual G. herbaceum or the hardy cotton bush G. arboreum produces fibres with a length of 1.8-2.2 cm. Long stapled varieties are cultivated mostly in Egypt and Peru. The middle stapled varieties from the USA, short stapled varieties in Asia G. barbadense is more susceptible to pests due to its long vegetation period G. hirsutum, ripens far quicker (some varieties after only 150 days).
All of the cotton varieties with coloured fibres, formed from crossings between wild varieties (from Peru) and crops, have provoked a certain interest in the natural textile processing industry. Until now, mostly brown, green and beige varieties had been cultivated.
Organic cotton production is most widely spread in the USA (about 4000 ha). Yet ecological cotton projects also exist in Egypt, Argentina, Brazil, Greece, India, Mali, Nicaragua, Paraguay, Peru, Tanzania, Turkey and Uganda.
- about 320 - 420 kg fibres (raw cotton)
- about 200 - 250 kg seed cakes or flour
- about 100 - 150 kg oil
- about 200 kg shells
- about 20 kg retained seeds
- about 40 kg dirt
The fibres (lint-fibres, lint) are used in the production of materials (threads, fabrics, etc.), whilst the linters (fluff separated from the cotton seeds) are used to produce cellulose fibres and other cellulose products, thick threads and stuffing materials, as well as being used for the production of paper.
Oil produced from the seeds can be used as edible oil, among other things. When oil is produced from the cotton seeds and pelleted feed used as fodder, care must be taken to remove the Gossypol that forms in the oil/cakes by heating it. Gossypol is poisonous to humans and animals with normal stomachs (e.g. pigs), only ruminants (e.g. cattle) can digest cakes containing Gossypol without a problem. The seed shells can be used as raw fodder for animals, as straw, dung or as fuel.
Low-grade oil is used in the manufacture of soap, lubricants, sulphonated oils and protective coatings. The seed cakes are used as fodder and manure. The seed shells can be used as raw fodder for animals, as straw, or as fuel.
The crop will not tolerate very heavy rainfall and, where grown as a rain-fed crop, the average rainfall is usually 800-1200 mm. Adequate, but not excessive, moisture is required for early vegetative growth. The first flowering period requires relative dryness to speed up formation of fruiting branches. An increase in moisture is required for boll setting and renewed growth, followed by dry weather for ripening and harvest. Sufficient soil moisture is essential during the flowering period.
In the tropics most cotton is grown by smallholders who sell their seed cotton to the ginneries. Ginneries may be privately, cooperatively or state owned.
Cotton can be grown on a variety of soils from light sandy soils to heavy alluvium and calcareous clays. Soils must be permeable to water and to roots to a depth of at least 100 cm, preferably over 150 cm, with pH 5.5-8.5. Cotton is one of the salt-tolerant crops.
In its early stages of growth, cotton requires an arid climate with a plentiful supply of water. Afterwards, the weather needs to be dry, especially after the capsules have opened, for if water can enters the capsules it will have a detrimental effect on the quality. The vegetation period generally lasts around 180-220 days (varieties such as G. hirsutum that mature rapidly can be harvested after only 150 days). Very high yields have been reported from the arid areas of the CIS and in Egypt using irrigation.
Because cotton loves the heat, yet is also highly susceptible to frost, temperatures of around 26-28°C are ideal for its development. Lots of sun has a very positive effect during the blossoming and fruit setting periods, in cases of 50% and more cloud during the vegetation period it makes little sense to plant cotton. Cotton cannot withstand shade. Cotton is also a short-day plant, and such conditions will accelerate its growth. The correct climatic conditions are generally found between the 28° northern and 47° southern latitudes.
A strong wind can suck the fibres out of the capsules and blow them away. Today's varieties are tolerant with regard to salinity (up to a salinity of 0.5-0.6%). The soil's pH value should be between 6 and 8. In addition, cotton also requires deep, well-drained and ventilated soil, in order to properly develop its system of tap-roots (resistance to drought).
The site's elevation plays a large role when planting coloured varieties of cotton, because the intensity of the sun's rays has a strong effect. At least the green varieties tend to bleaching when the intensity increases too much (Peru).
Seeds and shoots
The fertilisation process is generative. Most wild Gossypium varieties are perennial. Annual varieties are most generally used for cultivation, which conclude their development cycle during one single vegetation period. Local and regionally produced seeds should be used, which will have developed a tolerance or resistance against the pests most commonly found in the region. Because the sale of seeds is usually controlled by government authorities, it is important to try to acquire untreated seeds of the desired variety early enough.
During the last few years, hybrid seeds have been developed that provide high yields. Yet this method makes it impossible to use self-produced seeds from the crop, and a new supply of seeds must be bought for the next season.
Because cotton can be affected by various root and damping off diseases, in certain cases, it is worthwhile considering pre-treating the seeds. In contrast to conventional cotton crops, only micro-organisms, which work antagonistically, are used. In Egypt, for example, the seeds are treated with Bacillus subtilis, Gliocladium penicilloides and/or a suspension of trichoderma. In order to improve the nutrient availability for the young plants in India, the seeds are additionally treated with Azotobacter and bacteria strains capable of breaking down phosphorous.
Sowing methods
If the cotton is to be planted by machines, then the seeds need to be rid of the fluff that surrounds them (otherwise the seeds stick together). This is not necessary when the seeds are sown by hand. The temperature should not fall below 18°C. Temperature of 35°C is optimal. The seeds should be sown at a depth of maximum 5 cm.
The density depends upon the method utilised (manual or by machine). Bio-dynamic farmers in Egypt sow several seeds by hand every 20 cm into the prepared planting rows (distances between the rows 60-70 cm). Between 3 and 4 weeks later, the plants are inspected and all but the 2 strongest specimens removed. The plant population of rain-fed cotton of small-scale farmers in Tanzania is much lower. The farmers sow cotton by broadcasting the seeds on the flat land or in rows at a spacing of 90 cm between and 30 - 50 cm in the rows. Some farmers sow also on ridges with the same spacing.
Mechanical methods usually leave 70 cm (50-120 cm) between the rows and 20 cm (20-60 cm) distance between the seeds. When the harvest is done mechanically, varieties such as G. hirsutum that produce few branches are sown every 8-10 cm with a distance between the rows of 15-20 cm.
Cotton is either planted on flat soil, ridges or in furrows. Furrow drilling is employed mainly as a protection against quicksand. Ridges are used in soils, which are difficult to drain, and in regions with little rainfall, as this eases irrigation and facilitate the seeping in of the water into the soil. Its disadvantages are more difficult sowing and tilling of weeds. The cotton is sown in the lower third of a ridge in high-content soils and the upper third for low content soils. The seeds should be watered as soon as possible after sowing.
During the first 3 weeks, the shoots can offer little resistance against weeds, but this improves until the thick crop growth has no more problems in the area. For this reason, a suitable position in the crop rotation, suitable soil cultivation method and preparation of the seed beds should be taken care of to prevent an excessive growth of weeds during the early growth phases. On irrigated soils, irrigation is carried out prior to sowing in order that the weed seeds germinate and grow. The resulting growth of weeds can then be easily removed by appropriately cultivating the soil, before the cotton is sowed. The final soil treatment before planting should include the spreading of compost.
For cultivation done using animal-drawn (oxen or water-buffaloes) implements time requirements are: 15 animal days and person-days/ha, or by hand: 50 person-days/ha. By 2 or 4-wheeled tractors (150 kWh/ha).
The land should be prepared early and to a depth of at least 15 cm. To maintain soil organic matter, liberally apply or incorporate plant residues and animal manure during land preparation. Planting should be early, as soon as rainfall is adequate for the germination and growth of the crop. Cotton grown by smallholders is commonly planted with a delay, because the food crops are given priority. In hand planting, cotton is usually sown at a seed rate of 11-14 kg/ha and at a depth of about 25 mm with 3-6 seeds per hole in rows or ridges. Ridges are an advantage as they can be tied to conserve water under dry conditions and aid drainage under wet conditions. Thinning is done when the plants are 6-10 cm high, and two plants per hill are usually left. The optimum spacing depends on the size and fruitfulness of the plant permitted by local conditions. It also depends on the interactions between variety, soil and climate. The optimum spacing ranges from 80-20 to 100-40 cm, with one or two plants per hill. Plant densities may vary between 40,000 and 100,000 plants per ha, but are generally between 50,000 and 60,000 plants per ha.
Cotton intercrops such as maize, sorghum, beans, and peanut create a natural balance of pests, natural enemies, and weeds in the cotton field environment. Maize or tobacco planted in every 20 rows of cotton attracts African bollworm. Sunflower or cowpea sown in every 5 rows of cotton attracts moths when planted as trap crops. Castor bean (Ricinus communis) attracts caterpillars. Rice when rotated with mungbean and cotton disrupts the life cycles of pests attacking these crops. However, the timing of planting of intercrops, trap crops, and border crops should be planned to flower at the same time with cotton.
Yet this requires a successful plant protection management system. The farmer should consider the following:
- Which important infection agents are present in the region?
- Which preventative strategies he wishes to implement against them on his site?
- Which combating measures exist against a heavy infestation?
- Which permitted resources are available for organic systems?
- How these are applied?
- When it is correct time to apply them?
- Whom he can turn to in an emergency (advice)?
Selecting crop rotation
As cotton is not well compatible with itself, it is not advisable to have a larger ratio than 1/3 in the crop rotation. Other mallow plants (e.g. hibiscus, ocra) must be excluded from the rotation, or at least not planted on the same soil. It is also important to check that no cotton is grown on any of the neighbouring plots. On the whole, a diversified crop rotation works best.
Mixed crops with plants that act as a repellent
Mixed or strip cultivation with onions, garlic, chillies, chrysanthemums or hot peppers have proved their worth because of their repellent effect against, among others, bugs, white fly and cotton leafworm (Alabama argillacea). Rotted liquid manure can also act as a repellent (and be simultaneously used as a fertiliser).
Cultivation of trap crops
Trap crops manage to keep pests away from the cotton by offering a more attractive source of food. Strip cropping using lucerne (Medicago sativa L.) within the cotton plants is, for example, practised in the USA and Paraguay, in order to keep pests such as different bug species (Dysdercus spp., Lygus spp.), Helicoverpa spp., Spodoptera littoralis, Platyedra gossypiella and aphids away from the cotton.
Sowing sorghum before the cotton (on neighbouring plots) can help to build up a population of useful insects, which can then combat cotton pests when they appear at an early stage (e.g. aphids).
A similar strategy can be followed by planting Hibiscus esculentus against the pest Podagrica spp., planting Lablab niger L. against the pests Helicoverpa spp., Spodoptera littoralis and Bemisia tabaci, or nasturtium against Tetranychus cinnabarinus (these are based on experiences from Turkey and in Sudan). During 9 years of organic cotton growing in Tanzania the experiences have shown that the most important cotton pest Helicoverpa spp. can be controlled with sunflower as trap crop to such an extent that the threshold for an economic application of insecticides is not reached in most cases. The recommended practice is to sow one row of sunflower around the cotton plot as a living fence and one row of sunflower all 10 meters in the plot. The sowing time has to be very close to the cotton sowing so that the sunflowers will be in the flowering stage when the infestation period starts. The sunflowers attracts the moths of Helicoverpa spp. to lay their eggs. The caterpillars can feeding on the sunflower however without destroying the production of sunflower seeds. So the farmers can get an additional income from the sunflower seeds.
The caterpillars on the sunflowers show also the phenomenon of cannibalism so that they reduce their own numbers itself (Source: Dr. Braun, GTZ-IPM project, personal communication, 1997).
The positive effects of sunflowers are also shown in the results of a study that was carried out by an entomologist on behalf of the GTZ-IPM project in Shinyanga, Tanzania. The researcher found out that in organic cotton plots with sunflowers were up to ten times more useful ants compared with cotton plots without sunflower. It is known that these ants are reducing the eggs and larvae of the African bollworm (Helicoverpa armigera). (Source:Varela (1996) "Ants as mortality factors of the African bollworm Helicoverpa armigera in smallholder cotton fields in Tanzania"). A booklet about the natural enemies of the African bollworm names especially the ant species Myrmicaria and Pheidole as important. 'On sunflower, ants were observed to reduce bollworms by as much as 85%.' (Source: van den Berg, H. and Cook, M.J.W., (1993) 'African bollworm and its natural enemies in Kenya', P. 33, CAB International + NRI, International Institute of Biological Control, Kenya Station). Many contracted smallholders in Tanzania confirm the positive effects on the cotton yield by cultivating sunflower as trap crop and even many conventional neighbours in the region started copying this cheap and easy method of preventive pest control.
Pigeon peas (Cajanus cajan) can also be a useful trap crop for pests like Helicoverpa spp. but it is not so easy to synchronise the flowering stage of the trap crop with the infestation period of the pest. The local pigeon pea varieties in Tanzania start the flowering too late to be an efficient trap crop. Early maturing varieties or sowing of pigeon peas in the previous year could resolve this problem. In the bioRe India project the Pigeon peas are successfully used as trap crop in cotton.
Trap crops planted in autumn (e.g. maize) can be used in combination with a pheromone against hibernating boll weevils.
Leaving a strip of natural vegetation around the cotton plot can be useful against aphids and other pests.
Choosing a site
Cotton should be planted in healthy soil wherever possible. In principle, sites that are infested with weeds should not be sown with cotton, but first cultivated with an appropriate rotation crop in order to prepare it. Care should be taken that no cotton is planted in the neighbouring plots. Sowing time
The choice of when to sow plays an important role. Cotton sown too early will possibly become infested by the previous pest population. In Tanzania the late sown cotton is often attacked by African bollworm (Helicoverpa armigera) that developed on maize or sorghum plots. At the end of the season the risk of the late season pest cotton stainers (Dysdercus spp). is higher on late sown cotton while it is a minor problem on early sown cotton.
Mulching of harvest residues
Careful mulching of the remains of a cotton harvest can help prevent the survival of pests (e.g. P.gossypiella in seeds and boll weevil, Anthonomus grandis). In the case of heavy infestations with wilting diseases, such as bacterial blight (Xanthomonas malvacearum), anthracnose (Glomerella gossypii), verticillium-wilt (Verticillium alboatrum) or fusarium-wilt (Fusarium oxysporum) it is recommended to remove the residue and then apply compost.
Sufficient, balanced supply of nutrients
A plant that receives balanced nutrients is more vigorous, and therefore less susceptible to infestation. As already mentioned, supplying too much nitrogen will lead to an infestation by pests.
Choosing a variety
It is hereby important to choose varieties adapted to the site conditions, and that are resistant to, or can tolerate, the main pests. In addition, general varieties have proven their worth that matures quicker, thereby shortening the time span they can be infested. Gossypol-free are not so well suited to organic plantations because Gossypol (just like other terpenoide chemical compounds) has a repellent effect on certain insects (e.g. against Helicoverpa spp., Spodoptera spp. and Pectinophora spp.)
How easy or difficult it is to choose a suitable variety show the examples from Tanzania and India.
While in Tanzania there is only 1 cotton variety per production zone the bioRe farmers in India use 9 out of more than 50 different varieties according to their specific needs and preferences. It follows a list with the advantages and disadvantages of the varieties used in India, which shows the different aspects that can be important for the decision.
Planting of boundary areas
Planting 2-3 rows of trees or hedges along the boundaries provides a habitat for birds, improves climatic conditions and reduces the amount of water needed for cotton.
Checking the infestation level of cotton pests
In Tanzania the gtz-IPM Project has developed a method to check the infestation level of the key pest Helicoverpa armigera. The method is called 'scouting' and it works by counting the squared buds on 30 plants of 1 acre. If the number of squared buds comes up to 15 the economic threshold is reached and the farmers are advised to spray an insecticide. The organic farmers can apply an oily formulation of neem. This 'scouting' method works much faster than looking for the pests itself and helps to avoid many applications of insecticide (in organic and in conventional farming).
Light traps allow monitoring the start of pests moving into the cotton plot and at the same time reducing the number of moths laying eggs on the cotton plant. In Tanzania, traps are placed in the fields at rate of one per acre for about two hours after sunset. The reduction of moths can help to reduce the number of sprayings.
Direct combating measures
Direct methods of combating pests are also available for organic plantations, yet are only to be used in emergencies (and not as preventatives). It is necessary that the cotton, and any pests which may eventually develop, should be regularly inspected in order to be able to decide whether a direct method is to be used or not (see chapter above).
If the critical threshold is reached and there is an immediate threat for the cotton harvest the organic farmers need to have insecticides available that are allowed in organic farming. There are several botanical insecticides, which have prooved to be efficient against important cotton pests.
In India the bioRe farmers can choose among 3 commercial neem formulations and the self-made preparations made with crushed neem seeds. In Tanzania neem formulations imported from Kenya and another from India are commercially available for control Helicoverpa armigera. Against the late season pest Dysdercus spp. the farmers use a locally formulated Pyrethrum preparation with black wattle extract (Acacia mearnsii) as UV-light stabilisator.
Beside the neem products there are also some other plants that can be useful to produce botanical insecticides. In Tanzania the Ukiriguru Research Institute tested with promising results an emulsion of Jatropha curcas oil against Helicoverpa armigera. In Mali it has been tested against sorghum pests and a report from Malawi states. The oil and aqueous extract from oil (active principle probably phorbol ester) has potential as an insecticide, for instance in the control of 4 insect pests of cotton including cotton bollworm (Solsoloy 1995) and on pests of pulses, potato and corn.' (Malawi Agroforestry Extension Project Marketing & Enterprise Program Main Report, Publication No. 47, page 46, July 2002).
In West African countries like Bénin and Mali the farmers are experimenting with mixtures of plant extracts and other ingredients. Experiences from Bénin give the following recommendations for 1 ha:
3 sprayings during early season with a preparation of
1.5 kg pounded neem seeds in water, fermenting over night and then filtered
1 litre cow urine
20 papaya leaves
local soap diluted in water
total of 10 litres
Then later in the season the following preparation without cow urine in order to avoid excessive vegetative growth:
2 kg pounded neem seeds in water, fermenting over night and then filtered
5 cloves of garlic
20 papaya leaves
local soap diluted in water
total of 10 litres
Since 1999 organic cotton growers in Mali use a mixture of neem and 'Npeku' oil (Lannea microcarpa) as botanical insecticide. The preparation for 1 ha is done as follows:
- 500 g grounded neem seeds in 10 litres of water for 3 to 5 days and then filtered
- Add 40 to 160 ml of 'Npeku' oil (according to plant stage, see table below) and mix well to get an emulsion
Instead of the 'Npeku' oil the organic cotton farmers in Mali take also the oil of 'KOBI' (Carapa procera) in the same way.
Yellow traps, pyrethrum and also sulphur extracts do not work specifically enough (useful insects are also affected). For this reason, these preparations should only be used when absolutely necessary, and when no other alternatives are available.
Direct methods of combating pests are also available for organic plantations, yet are only to be used in emergencies (and not as preventatives). It is necessary that the cotton, and any pests which may eventually develop, should be regularly inspected in order to be able to decide whether a direct method is to be used or not (see chapter above).
|
Bollworms (Helicoverpa armigera, Earias spp and Pectinophora gossypiella) Bollworms are among the most serious cotton pests. They feed in the bolls, damaging lint and seed and causing considerable reduction in yield and quality. The main bollworms are the African bollworm (Helicoverpa armigera), spiny bollworm (Earias spp) and the pink bollworm (Pectinophora gossypiella). Caterpillars of the false codling moth (Cryptophlebia leucotreta) are sometimes found boring in the bolls.
| 
African bollworm
African bollworm feeding on okra. © A. M. Varela, icipe |
|
What to do:
|
- Acland, J.D. (1980). East African Crops. An introduction to the production of field and plantation crops in Kenya, Tanzania and Uganda. FAO/Longman Publication. ISBN: 0 582 60301 3.
- Bohlen, E. (1973). Crop pests in Tanzania and their control. Verlag Paul Parey. Federal Agency for Economic Cooperation. ISBN: 3-489-64826-9.
- CAB International (2006). Crop Protection Compendium, 2006 Edition. Wallingford, UK www.cabi.org
- Eyhorn, F., Ratter, S.G., Ramakrishnan, M. (2005). Organic Cotton Crop Guide - A manual for practitioners in the tropics. Research Institute of Organic Agriculture (FibL). www.fibl.org
- Kabissa JCB, (1989). Evaluation of damage thresholds for insecticidal control of Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae) on cotton in eastern Tanzania. Bulletin of Entomological Research, 79(1):95-98.
- Naturland e.V. (2004). Organic Farming in the Tropics and Subtropics. Exemplary description of 20 crops. Cotton. www.naturland.de
- Nyambo, B. T., Varela, A. M., Seguni, Z., and Kirenga G. (2003). Integrated Pest Management in Tanzania. In Integrated Pest Management in the Global Arena. Eds. K. M. Maredia, D. Dakouo and D. Mota-Sanchez. Chapter 13. PP. 145-155. ISBN: 0-85199-652-3.
- Online Information Service for Non-Chemical Pest Management in the Tropics (OISAT) www.oisat.org
- Puri, S. N., Sharma, O. P. Murthy, K. S. and Raj, Sheo. (1998). Hand Book on Diagnosis and Integrated Pest Management of Cotton Pests. National Centre for Integrated Pest Management, Pusa Campus, New Delhi, India. NCIPM.
- Schmutterer, H. (1978). Cotton pests in the Philippines. GTZ. ISBN: 3-88085-064-X.
 Back
Back
