
|
Sorghum
Scientific name:
Sorghum bicolor
Order/Family:
Cyperales: Poaceae
Local names:
Mtama (Swahili)
Pests and Diseases:
African armyworm
African bollworm
African maize stalkborer
Anthracnose
Aphids
Birds
Charcoal rot
Covered kernel smut
Crazy top downy mildew
Cutworms
Damping-off diseases
Ergot
Head bugs
Head smut
Leaf blight
Loose kernel smut
Maize dwarf mosaic virus
Other bugs
Purple witchweed
Rust
Sedges
Shoot fly
Sorghum midge
Spotted stemborer
Storage pests
Termites
Chafer grubs
|
African armyworm (Spodoptera exempta)
It is an occasional but seriously destructive pest. It causes serious damage to young plants in years of armyworm outbreaks. The leaves are eaten away often leaving only the base of the stem.
- Monitor regularly field margins, low areas where plants have lodged, beneath plant debris around the base of plants, on the ground, and underneath the plant leaves. Check daily young crops if conditions are known to be favourable to the pest outbreaks.
- Spray Bt or botanicals such as neem and pyrethrum extracts. Spray when caterpillars are small. Once caterpillars are mature (about 3 to 3.5 cm long) they may have cause serious damage and it may no longer be economical to treat the crop For more information on neem click here., on pyrethrum click here , Bt click here
- Conserve and encourage natural enemies. For more information on natural enemies click here.
- Practise field sanitation. For more information on field sanitation click here.

© A.M. Varela, icipe
African bollworm (Helicoverpa armigera)
It causes damage to sorghum by eating the seeds. Damage is particular serious on compact headed sorghums. The caterpillars appear on the ear heads when the grains are in the milkripe stage. These caterpillars also feed on whorl leaves. A heavy infestation can cause an almost complete loss of yield.
- Inspect field once or twice a week after sorghum begins to bloom. Check for presence of caterpillars by shaking heads over a bucket or sweep net.
- If necessary spray with Bt or neem extracts. Good spray coverage is very important, particularly in partially opened heads or in varieties that have tight heads where young caterpillars are well protected. For more information on neem click here. For information on Bt click here
- Handpick and destroy the caterpillars. This helps when their numbers are low and in small fields.

© A.M. Varela, icipe
Anthracnose (Colletotrichum graminicola)
The anthracnose fungus damages foliage and stems of grain sorghum. On susceptible hybrids, the stem holding the head (peduncle) becomes infected and a brown sunken area with distinct margins develops. When infected stems are cut lengthwise with a knife, one can see that the fungus has penetrated the soft pith tissue and caused brick-red discolourations. This peduncle infection inhibits the flow of water and nutrients to the grain causing poor grain development.
The fungus also invades individual grains and the small branches of the panicle. Rapid and severe yield loss can result from panicle and peduncle infections. Leaf lesions are small, elliptical to circular, usually less than 0.9 cm in diameter. These spots develop small, circular, straw-coloured centres with wide margins that may vary in colour from reddish to tan to blackish purple. The spots may coalesce to form larger areas of infected tissue.
- Use resistant hybrids.
- Rotate with non-cereals preferably with pulses.
- Good management of crop residues.

© Courtesy EcoPort (http://www.ecoport.org): J.A. Frowd
The sorghum aphid (Melanaphis saccari) and the maize aphid (Rhopalosiphum maidis)
These are common on sorghum. The sorghum aphid is light yellow in colour, and the maize aphid is dark green to bluish-green in colour. These aphids are often found sucking on ear heads or on the underside of leaves. They produce large quantities of honeydew, which enable black sooty moulds to grow. Attacked plants sometimes are stunted, leaves dry up and yield is reduced. Young plants suffering from drought stress may be killed. Aphids can be a problem during dry periods. Heavy aphid infestations on sorghum at the booting and heading stages seriously reduce both grain quality and yield. The maize aphid transmits the maize dwarf mosaic virus to sorghum.
Adults are small, 1-4 mm long, soft-bodied insects.
- Conserve natural enemies. Parasitic wasps and predatory insects, including lady bird beetles, damsel bugs, lacewings, and hover fly larvae are important in natural control of aphids.

© www.inra.fr
Birds
Birds are one of the most important pests of sorghum. They are capable of causing heavy losses. In Africa the most notorious species is Quelea quelea and is found in the Sahel region, from Senegal in West Africa through to Sudan, Uganda, Kenya, Southern Tanzania, Malawi, Zambia and South Africa.
- Damaging birds are mainly controlled by scaring them away from the sorghum fields and attacking their nesting sites. But not all birds are harmful. Some are also important predators and prey on insect pests of crops.

© Courtesy EcoPort (http://www.ecoport.org) : C.Elliott.

Red-Billed…

Red-Billed…

Red-billed…
Charcoal rot (Macrophomina phaseolina)
Grain sorghum plants affected by the charcoal rot fungus fail to fill grain properly and may lodge in the latter part of the season. Infected stalks show an internal shredding at and above the ground line. This can be observed by splitting the stalk and noting the deteriorated soft pith tissue leaving the tougher vascular strands. Fungal structures (sclerotia) can be observed in the affected tissue, which appears as though it has been dusted with black pepper. Another type of stalk rot (Pythium sp. and Fusarium sp.) may show the shredded condition but the black specks (sclerotia) will be lacking.
Conditions under which charcoal rot is favoured include stressful hot soil temperatures and low soil moisture during the post-flowering period. Host plants are usually in the early-milk to late-dough stage when infection occurs. The fungus is common and widely distributed in nature.
- Avoid moisture stress.
- Manage properly crop residue.
- Rotate crop with non-cereals. Legumes are also susceptible to the disease.
- Avoid excessive plant populations.
- Balance nitrogen and potassium fertility levels.
- Grow drought-tolerant, lodging-resistant hybrids.
Covered kernel smut (Sporisorium sorghi)
The disease destroys all kernels in a head and replaces them with a cone-shaped gall or may affect only portions of a panicle. At harvest time, these galls are broken and spores contaminate the outer surface of other kernels.
- Use of certified disease-free seed.
- Plant resistant hybrids.

© Courtesy EcoPort (http://www.ecoport.org): J. Kranz
Crazy top downy mildew (Sclerophthora macrospora)
This fungal disease can be troublesome in low lying areas that become flooded. Infected plants have thick, stiff, twisted, pale green leaves with bumpy surfaces. The leaves often turn downward, and the plants produce many shoots or suckers giving a bunchy appearance. Infected plants do not produce heads or produce a proliferation of leafy tissue in place of the head. Wild and cultivated grasses can serve as sources of infection.
- Plant resistant varieties.
- Remove diseased plants from the field.
- Rotate with non-cereals.
- Avoid excess soil moisture in the field.

© Courtesy EcoPort (http://www.ecoport.org): J. Kranz
Cutworms (Agrotis spp and Spodoptera spp.)
Several species damage sorghum. They may cut off young plants at or slightly below the soil surface. Some feed on above-ground plant parts, and others feed on the roots. Plants with severed stems die, leaf feeding by cutworms causes ragged leaves and feeding on roots may kill young plants or stunt older plants.
Chaffer grubs (Schizonycha spp.) also feed on roots and may kill very young seedlings. Stand loss can occur within 10 days after plants emerge in severely infested fields.
- Harrow and plough field and remove weeds well ahead of planting the crop in the field. Ploughing exposes caterpillars to predators and to desiccation by the sun. If the field is planted soon after land preparation some cutworms may be alive and attack the new crop.
- Inspect soil carefully when preparing land for planting for the presence of cutworms or white grubs.
- Monitor damage by counting damaged and freshly cut young plants.
- Collect and destroy cutworms. Cutworms are found in the soil close to damaged plants at day time. Monitor for cutworms at dawn.

© A.M. Varela, icipe

Cutworm

Whitegrub
Damping-off diseases
They are caused by various fungi. Seed may be attacked by one or more seed-borne or soil-borne pathogens prior to either germination or emergence. This usually occurs when conditions are not optimum for plant development, such as in poorly-drained, cold, wet soils, or in very dry, crusted soils. Sorghum seedlings are very delicate during the emergence period and are slow to establish a permanent root system. As a result, sorghum depends upon its primary, temporary root system for a period longer than many other crops. Under less than optimal growing conditions, this primary root system is extremely vulnerable to soil-borne pathogens. This is when damping-off in a field is most visible and damaging. Affected seedlings are unthrifty and may show yellowing, wilting and death of leaves. Roots of diseased plants may be discoloured (whitish grey to pinkish brown) and rotted.
- Use certified disease-free seeds.
- Avoid planting in poorly drained soils.

© A.A. Seif & A.M. Varela, icipe
Ergot (Claviceps sorghi)
Cream to pink sticky droplets "honeydew" ooze out of infected florets on panicles. The droplets dry and harden, and dark brown to black sclerotia (fungal fruiting bodies) develop in place of seeds on the panicle. Sclerotia are larger than seed and irregularly shaped, and generally get mixed with the grain during threshing. Conditions favouring the disease are relative humidity greater than 80%, and 20 to 30°C. The sclerotia falling on the soil or planted with the seed germinate when the plants are flowering. They produce spores that are wind-borne to the flowers, where they invade the young kernels and replace the kernels with fungal growth. The fungal growth bears millions of tiny spores in a sticky, sweet, honeydew mass. These spores are carried by insects or splashed by rain to infect other kernels.
- Plant resistant varieties, where available.
- Remove affected panicles.
- Avoid planting seeds from infected panicles.
- Plough deep.
- Rotate with non-cereals preferably with pulses.
- Practise good field sanitation

© Courtesy EcoPort (http://www.ecoport.org): G. Odvody
Head bugs (Calocoris angustatus and Eurystylus oldi)
Nymphs and adults of the head bug (Calocoris angustatus) and the African head bug (Eurystylus oldi) feed on developing kernels as panicles emerge from the boot. Head bugs are small (3 to 5 mm long, and about 1 mm wide) and variable in colour from yellowish green (C. angustatus), or pale brownish-yellow to dark brown with red markings (E. oldi). Females insert long, cigar-shaped eggs between the glumes or anthers of sorghum florets. Eggs usually hatch in less than a week. Nymphs and adults suck juice from developing kernels as panicles emerge from the boot. Kernels attacked early in development are shrivelled, small, and off-coloured, resulting in yield loss. Bug-damaged kernels become infected by secondary pathogens that further deteriorate grain quality. Feeding punctures are visible on older kernels. The life cycle is completed in about three weeks. At least two generations feed on the same crop when panicles in the field do not mature at the same time.
- Conserve natural enemies. Assassin bugs and lygaeid bugs prey on ear head bugs. For more information on natural enemies click here.
- Selection of varieties. Open panicles are less affected than compact panicles.
- Resistant varieties. Some sorghum varieties are resistant to bugs.
- Timing of planting. Damage is less severe when kernels develop during dry periods.
Head smut (Sporisorium reilianum)
This disease is characterised by the large, dark-brown smut galls that emerge in place of the panicle. The gall is first covered with a whitish membrane, which soon breaks and allows spores to be scattered by the wind. Plants become infected while in the seedling stage but evidence of infection is not apparent until heading time. The smut gall produces thousands of spores, which become soil-borne and initiate systemic infection of seedlings in subsequent years. Different races of the fungus exist which may result in a sorghum hybrid being resistant in one area but not another.
- Plant resistant hybrids to avoid losses.
- Use certified disease-free seed.
- Rotate with non-cereals.
- Plough deep.

© CAB International
Leaf blight (Helminthosporium turcicum)
Leaf blight (Helminthosporium turcicum)
Attacks sorghum, Sudan-grass and maize. The causal fungus is carried on the seed and also lives in the soil on dead or decaying plant material. It may cause seed rot and seedling blight, especially in cool and excessively moist soil. Seedlings then can become infected readily and may either die or develop into stunted plants. Small reddish-purple or yellowish-brown spots usually develop on the leaves of infected seedlings.
The spots may join to kill large parts of the leaves, which then dry to the extent that severely affected plants look as if they have been burnt. A greenish, mould-like growth of spores develops in the centre of the leaf spots during warm, humid weather. The spores are spread by wind or rain and infect other leaves and plants. Under warm, humid conditions the disease may cause serious damage by killing all leaves before plants have matured.
- Plant resistant varieties.
- Use certified disease-free seeds.

© Courtesy EcoPort (http://www.ecoport.org): D.C. Nowell
Loose kernel smut (Sphacelotheca cruenta)
It attacks all groups of sorghum including Sudan-grass and Johnson grass. Galls formed by loose kernel smut are long and pointed. The thin membrane covering the galls usually breaks soon after galls reach full size. The dark-brown spores contained in the galls are wind-blown away leaving a long, dark pointed, curved structure (called columella), in the central part of the gall. As in covered kernel smut, the spores of the fungus are carried on the seed and germinate soon after the seed is planted and invades the young sorghum plant. It continues to grow unobserved inside the plant until heading, when the long pointed smut galls appear in the heads in place of normal kernels. Unlike covered kernel smut, this disease stunts the infected plants and often induces abundant side branches.
- Certified disease-free seeds.
- Plant resistant varieties.
- Control weeds.
- Rotation with non-cereals.
- Practise good field sanitation.

© Courtesy EcoPort (http://www.ecoport.org): J. Kranz
Maize dwarf mosaic virus (MDMV)
Maize dwarf mosaic is a virus disease that occurs over all the sorghum producing areas. Its ability to cause damage is dependent on the presence of an over-seasoning virus host (mainly Johnson grass), aphid populations to facilitate virus transmission and the susceptibility of the varieties being grown. Affected plants have mottled (light green blotches) terminal leaves. These alternate light- and darker-green areas in the leaf can be more easily seen when held between the viewer and a light source. Observers should always look at the newest leaves for the most severe symptoms. Highly susceptible hybrids are stunted with chlorotic symptoms in the upper leaves and suffer significant yield losses. Some hybrids produce a red leaf symptom when plants are infected and when night temperatures are below 13° C.
- Use resistant or tolerant hybrids.
- Control Johnson grass in and around the field.
Other bugs
A number of bugs feed on the milkripe sorghum grains: shield bugs including stink bugs (Nezara viridula, Acrosternum spp), Mirperus jaculus, Riptortus dentipes, Lygus bugs, blue bug (Calidea degrii) among others. The bugs puncture the seeds and suck the contents. Feeding punctures remain as dark spots on the testa. The seed weight is reduced; the rate of germination may be depressed. Sorghum is most susceptible to bug damage during the milk and soft dough stage. Injury normally is not damaging from hard dough to maturity. The damage is only of economic importance when bugs are present in large numbers.
- Use neem-based pesticides. Reportedly they reduce feeding by green shield bugs. For more information on neem click here.
- Check for bugs by beating or shaking panicles over a sweep net or bucket.

© Russ Ottens, University of Georgia, Bugwood.org
Purple witchweed (Striga hermonthica)
The parasitic weed Striga is a major pest of sorghum, particularly in Africa, where severe infestations can lead to land being abandoned. Striga attaches itself to sorghum roots depriving it of nutrients and preventing them from establishing and growing properly.
- Striga can be controlled manually by vigorous uprooting before it produces seeds and/or by intercropping sorghum with fast spreading legumes which deprives the weed of sunlight and exude chemical substances the reduce striga growth. Desmodium for instance has been shown to depress striga almost completely. Considerable efforts have been dedicated to develop resistant varieties. Some of the varieties/lines developed or identified with resistance in Africa are: "Dobbs", "SAR 1" to "34", "L.1872, "ICSV 1002", "SRN", "Framida" and "IS938" (ICRISAT).

© Courtesy EcoPort (http://www.ecoport.org): David C. Nowell
Rust (Puccinia purpurea)
Rust appears on leaves as small raised pustules or blisters that rupture and release many reddish-brown spores. These pustules occur on both the upper and lower leaf surfaces. This disease usually appears when plants near maturity and infection is confined primarily to mature leaves. Grain yield losses are usually not serious and occurrence of the disease is sporadic. Forage sorghum yields may be affected most. The rust fungus also attacks Johnson grass and over-seasons on this host.
- Use resistant varieties.
- Rotate with non-cereals.
- Control weeds.

© Courtesy EcoPort (http://www.ecoport.org): D.C. Nowell
The sorghum shoot fly (Atherigona soccata)
It is the most important pest of sorghum at the seedling stage. The adult is similar to the housefly, smaller in size (3-5 mm long), and greyish in colour, and abdomen yellow with brown patches. The larvae or maggots are yellowish or whitish in colour, up to 8 mm long. The fly lays eggs either at the base of young shoots near soil surfaces, or in older plants, on the leaves. The maggots crawl inside the sheath and bore into the heart of the young shoot killing the growing point and the youngest leaf, which turns brown and withers. This damage is known as "dead heart". When good growing conditions prevail the young plants are usually able to compensate the damage by producing new tillers, which may partly escape attack, but later the ripening of the ear heads will be unequal. In weak plant repeated infestation may cause serious losses. Sometimes the damage is so severe that many seedlings die and the field has to be replanted. Older plants (over 30 days after seedling emergence) are generally not damaged by the shoot fly. However, when shoot flies are abundant (during the rainy season under moderate temperatures and high humidity) older plants may be attacked, but they do not produce the dead-heart symptoms. Instead, the damaged leaf becomes thin and papery, and wraps around the other leaves. As a result, the plants may fail to grow normally. Late infestations may also damage the panicle in the formative stage, resulting in rotting or drying up of a portion of the panicle affected by shoot fly damage.
- Conserve natural enemies. A range of natural enemies attacks the sorghum shoot fly: parasitic wasps attack eggs and maggots and predators cause high mortality of eggs. In particular, several species of spiders are important predators on eggs. For more information on natural enemies click here
- Field sanitation. Crop residues should be collected and destroyed after harvest to reduce carry-over from one season to another.
- Plant resistant or tolerant varieties where available: Trials in Southern Africa has shown significant differences in resistance to shoot fly damage among varieties tested. Although the level of resistance in many of the sorghum varieties was low, several varieties with moderate levels of resistance were identified. Varieties "Pirira-1" and "Pirira-2" were the most resistant across seasons (van den Berg et al, 2005). In Eastern Africa, varieties "Serena" and "Seredo" showed high levels of recovery following shoot-fly damage (CABI; ICRISAT).
- Early sowing make often possible to have the period of vulnerability (seedling stage) over by the time the flies emerge.
- Uniform sowing of the same variety over large areas with the onset of rains reduces the damage by sorghum shoot fly.
- High seeding rates helps to maintain optimum plant stands and reduce shoot fly damage. There are reports that shoot-fly damage is higher when plant densities are low (CABI, 2000).
- Balanced fertiliser application. Application of fertiliser has been related to lower damage by shoot-fly possibly by increasing plant vigour. However, shoot-fly damage has been found to be greater in plots treated with cattle manure. This may have been due to the attraction of shoot flies to the odours emanating from the organic manure.
- Intercropping. It has been shown that shoot fly damage is reduced when sorghum is intercropped with leguminous crops.
- Fallowing and a closed season reduce the carryover and build-up of the shoot fly from one season to the next. These practices have been successfully used to reduce shoot-fly damage at ICRISAT. However, they may not practical for small-scale growers due to the shortage of land.

© Courtesy EcoPort (http://www.ecoport.org): Georg Goergen

Shoot fly

Sorghum sh…
The sorghum midge (Stenodiplosis sorghicola)
It is reported as one of the most important pests of sorghum in some countries, whilst in others (e.g. Ghana) is considered a sporadic pest. Nearly 30% of sorghum grain was damaged by sorghum midge in 1990 in western Kenya. In southern Africa, there are reports of 25% of sorghum grain damaged by sorghum midge (CABI, 2000). The adults are small (3 mm long), deep-red midges, with transparent wings. Eggs are laid in the flowering heads. The small orange larvae feed in the developing seed. Attacked seeds become shrunken and flat resulting in empty or "chaffy heads" as shrivelled grains fail to develop. The larva pupates inside the spikelet, and before adult emergence, the pupa wriggles its way to the tip of the spikelet. After adult emergence, the pupal case remains attached to the chaffy spikelet. Thus, damaged panicles have small, transparent midge pupal cases attached to the tip of the damaged spikelets.
- Synchronised planting. Epidemics of sorghum midge damage are common within an area, when sorghum is not planted at the same time, or different cultivars are planted that do not mature at the same time. Although landrace varieties often flower uniformly, high-yielding, early-flowering cultivars often do not. Sorghum that is planted and flowers later than normal is exposed to sorghum midge for a longer period and can suffer severe damage.
- Planting density and thinning. Midge damage is reported to be higher in crops with low plant density (CABI, 2000).
- Selective removal of alternative hosts. Wild species of sorghum (for example, S. halepense and S. sudanense) act as alternative hosts for sorghum midge. Midge populations build up early in the season on wild species of sorghum and infest the sorghum crop later in the season. Removing these alternative hosts from the vicinity of the sorghum crop can reduce the rate of multiplication of sorghum midge populations. However, wild hosts also sustain the natural enemies, and thus may help in increasing the role of natural enemies in population suppression.
- Field sanitation. Crop residues should be collected and destroyed to reduce the carryover of larvae in the chaffy spikelets from one season to another.
- Fallowing and close season. Fallowing reduces the carryover and build-up of midge populations from one season to the next. However, this is not a feasible practice for smallholders due to the shortage of land.
- Use tolerant or resistant varieties where available. High levels of host-plant resistance are available for sorghum midge. In India, varieties SPH 837 and Pratap Jowar 1430 are claimed to be tolerant to the midge damage (www.nrcjowar.res.in/aicsip2005/achievements_udaipur.pdf). Also in India, variety "DSV-3" is claimed to resistant to midge damage and is recommended to be planted in midge endemic areas (www.uasd.edu/research.htm).
- Crop rotations. Sorghum is generally rotated with cotton, groundnuts, sunflowers or sugarcane. This may reduce the damage caused by the sorghum midge.
- Mixed cropping. Damage by the sorghum midge is reduced when sorghum is intercropped with leguminous crops (CABI, 2000)

© Keith M. Harris from the Crop Protection Compendium, 2007 Edition. CAB International, Wallingford, UK, 2007.
Stemborers: Spotted stemborer (Chilo partellus)
Sorghum is attacked by several species of stemborers. The most important species include the spotted stalkborer (Chilo partellus), the pink stalkborer (Sesamia calamitis) and the maize stalkborer (Buseola fusca).
The feeding activity of the caterpillars inside the stems causes stunted plant growth, sterile or poorly developed ear heads. Plants may dry and die if the infestation is severe.
- Early planting to ensure maximum pest escape.
- Use resistant varieties.
- Habitat management. Intercropping sorghum with pulses (cowpeas, groundnuts) in alternate rows, may reduce stemborer incidence by 20-30%.
- Sanitation (destruction of crop residues, volunteer plants and alternative hosts). Crop residues (stalks and other residues) should be destroyed after harvest through burning the stalks, or fed to livestock. However, burning of crop residues may not be practical in communities where soil fertility is low and no fertilisers are used since crop residue is the only source of organic matter.
- Biological control. Recent research work on stemborers has been focussing on the introduction exotic parasitoids in countries where Chilo partellus is wide spread.

© Courtesy EcoPort (http://www.ecoport.org): Courtesy EcoPort
Storage pests
Sorghum is very susceptible to damage by storage pests, the main ones being greater grain weevils, in particular the rice weevil (Sitophilus oryzae), the flour beetle (Tribolium castaneum) and the grain moth (Sitotroga cerealella). Heavily attacked grain loses much of its content and become unfit for sale and consumption.
- Damage can be minimised by drying grain adequately before storage. Cultivars with hard grain also suffer less damage.

© Courtesy EcoPort (http://www.ecoport.org): Food Agency and Ministry of agriculture, forestry

Rice weevi…

Grain moth
| General Information and Agronomic Aspects | Information on Pests and Weeds | |||
| Information on Diseases | Information Source Links |
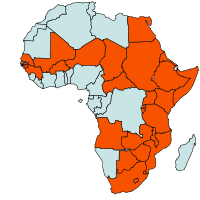 |
| Geographical Distribution of Sorghum in Africa |
Sorghum is perhaps the world's most versatile crop. Some types are boiled like rice, some cracked like oats for porridge, some "malted" like barley for beer, some baked like wheat into flatbreads, and some popped like popcorn for snacks. A few types have sugary grains and are boiled in the green stage like sweet corn. The whole plant is often used as forage, hay, or silage. The stems of some types are used for building, fencing, weaving, broom making, and firewood. The stems of other types yield sugar, syrup, and even liquid fuels for powering vehicles or cooking meals. The living plants are used for windbreaks, for cover crops, and for staking yams and other heavy climbers. The seeds are fed to poultry, cattle, and swine. (Lost Crops of Africa, Vol I, 1996) Sorghum plays an important role as a food security crop especially in semi arid lands of Kenya. It can survive drought conditions for some weeks by rolling up its leaves and thus decreasing transpiration. Please also check KARI update: "Sorghum helps provide better food security", July 1997, available in English on http://www.kari.org/ENGLISH/Sorghumfood.htm (click to follow link)
Nutritive Value per 100 g of edible Portion
| Raw or Cooked Sorghum | Food Energy (Calories / %Daily Value*) |
Carbohydrates (g / %DV) |
Fat (g / %DV) |
Protein (g / %DV) |
Calcium (g / %DV) |
Phosphorus (mg / %DV) |
Iron (mg / %DV) |
Potassium (mg / %DV) |
Vitamin A (I.U) |
Vitamin C (I.U) |
Vitamin B 6 (I.U) |
Vitamin B 12 (I.U) |
Thiamine (mg / %DV) |
Riboflavin (mg / %DV) |
Ash (g / %DV) |
| Sorghum | 339 / 17% | 74.6 / 25% | 3.3 / 5% | 11.3 / 23% | 28.0 / 3% | 287 / 29% | 4.4 / 24% | 350 / 10% | 0.0 IU / 0% | 0.0 / 0% | - | 0.0 / 0% | 0.2 / 16% | 0.1 / 8% | 1.6 |
Sorghum also tolerates water logging and can be grown in areas of high rainfall. It is, however, primarily a plant of hot, semi-arid tropical environments with rainfall from 250 mm that are too dry for maize but performs best with more than 900 mm annually. It is also grown widely in temperate regions and at altitudes of up to 2500 m in the tropics. Sorghum tolerates a wide range of temperatures. Sterility can occur when night temperatures fall below 12-15°C during the flowering period. Sorghum is killed by frost.
Sorghum can be grown successfully on a wide range of soil types. It is well suited to heavy clay soils (vertisols) found commonly in the tropics, where its tolerance to water logging is often required, but is equally suited to light sandy soils. It tolerates a range of soil pH from 5.0-8.5 and is more tolerant to salinity than maize. It is adapted to poor soils and can produce grain on soils where many other crops would fail.
Production zones in Kenya and recommended sorghum varieties and their major characteristics:
| Eco-zone and area | Variety | Maturity months | Grain colour | Yield potential
(90kg bags/acre) |
Special attributes |
| Moist-mid-altitude
Busia, Siaya, Kakamega, Kisumu, Homabay, Kuria, Migori, Coffee zones of Meru, Embu and Nyeri Districts |
"Serena" "Serodo" "KARI/Mtama1" |
3.0 3.5 3-3.5 |
Brown Brown White |
12 12 15 |
Wide adaptability Tolerant to striga Wide adaptability Tolerant to stem borers. Attractive to birds |
| Semi-arid low lands
Machachos, Kitui, Makueni, Mwingi, Lower Embu and Tharaka Nithi, Kajiado, Parts of Rift Valley, Parts of North Eastern Provinces |
"IS76" "KARI/Mtama1" "KARI/Mtama2" "Gadam" "Serena" "Serodo" |
3 3-3.5 3.5 3.5 3 3.5 |
White White White Greyish Brown Brown |
10 15 15 8-20 12 12 |
- Tolerant to stem borers. Attractive to birds Resistant to birds Tolerant to stem borers, shoot fly and foliar diseases Wide adaptability. Tolerant to striga Wide adaptability |
| Cold semi-arid Highlands
Nakuru, Baringo, Laikipia, Naivasha, Narok, Parts of Koibatek, Taita Taveta |
"E 1291" "E 6518" "BJ28" |
7 8 7 |
Brown Brown Brown |
12 15 12 |
Dual purpose. Good beverage quality Dual purpose. Good beverage quality Dual purpose |
| Humid Coast
Lamu, Kilifi, Taita Taveta, Kwale, Mombasa |
"Serena" "Serodo" "KARI/Mtama1" "Gadam" |
3 3.5 3 3 |
Brown Brown White Greyish |
12 12 15 8-20 |
Wide adaptability Wide adaptability Tolerant to stem borers. Attractive to birds Tolerant to stem borers, shoot fly and foliar diseases |
- "Epuripur": This is a white seeded variety, resistant to shoot fly and stem borers but susceptible to bird damage. It yields about 2.5 - 3.0 tons per ha. Grains are sweet and can be used for food, baking and brewing.
- "Sekedo": It is a dwarf variety (100 cm) with brown-red seeds. It is tolerant to stem borers and moderately resistant to shoot fly. It is recommended for food and feeds. It matures in 100 days with a yield potential of 4 - 5 tons per ha
- "Seredo" (variety characteristics as in Kenya).
- "Serena" (variety characteristics as in Kenya).
- "Seredo" (variety characteristics as in Kenya).
- "Serena" (variety characteristics as in Kenya).
- "Macia" ("SDS 3220") white grain; early maturing (80 days); yield potential 4t/ha
- "Pato" ("SDS 2293-6") grain colour cream/yellow white mottled; early maturing (80 days); yield potential 4 t/ha.
- "Tengemeo" ("2KX17/B/1") grain colour creamy white; early maturing (80 days); very good drought resistant; attractive to birds; resistant to major diseases and insect pests; good brewing quality; good storage; yield potential 4 t/ha.
- "Lugugu" (Landrace; white grain).
- "Udo Msonga" (Landrace; brown grain).
- "Msumbji" ("IS7173") (Landrace; high aluminium tolerance).
- "PN3" white grain; high grain yield (4 t/ha); good storage; very good brewing quality; insect and disease reaction poor; poor bird resistance.
Subsistence farmers rarely apply fertiliser, as responses depend on moisture availability, which is usually very uncertain. Under more favourable conditions, farmyard manure is used with advantage, but even so the quantities used are usually below optimum. Optimally sorghum needs the availability of about 20 kg N/ha and 20 kg P/ha at planting time, which can be supplied by alternate cropping with legumes and application of compost or manure. Also intercropping with legumes is recommended with grain legumes such as beans, cowpeas, pigeon peas and green gram. Manure and compost improve organic matter content of the soil, soil moisture retention ability and soil structure. Manure can be broad cast in the field or applied in planting furrows and mixed with soil before seeds are planted. The standard farm wheelbarrow when full holds approximately 25 kg of dry manure/compost. At a low rate, two wheel barrows are enough for a 10 m by 10 m area. This translates into 200 wheelbarrows or 5 tons/ha. When aiming for high rate apply 400 wheelbarrows or 10 tons per ha.
The crop is usually weeded by a combination of inter-row cultivation with animal-drawn implements and hand weeding within rows. Thinning is carried out at the same time as hand weeding, or at intervals during the crop cycle, particularly where thinnings are used to feed livestock. Gapping by transplanting thinnings is encouraged when thinning is done within 2 weeks after emergence and when the soil is moist.
In bimodal rainfall zones of semi-arid lowlands in Eastern province sorghum is planted in short rains (October- November). When the crop is mature, it is harvested in February and immediately ratooned to take advantage of the long rain season which starts in mid-March. To achieve good yields, the crop is thinned to 2-3 tillers per hill. Weeding and other management practices are done as for a newly sown crop.
|
The sorghum shoot fly (Atherigona soccata) It is the most important pest of sorghum at the seedling stage. The adult is similar to the housefly, smaller in size (3-5 mm long), and greyish in colour, and abdomen yellow with brown patches. The larvae or maggots are yellowish or whitish in colour, up to 8 mm long. The fly lays eggs either at the base of young shoots near soil surfaces, or in older plants, on the leaves. The maggots crawl inside the sheath and bore into the heart of the young shoot killing the growing point and the youngest leaf, which turns brown and withers. This damage is known as "dead heart". When good growing conditions prevail the young plants are usually able to compensate the damage by producing new tillers, which may partly escape attack, but later the ripening of the ear heads will be unequal. In weak plant repeated infestation may cause serious losses. Sometimes the damage is so severe that many seedlings die and the field has to be replanted. Older plants (over 30 days after seedling emergence) are generally not damaged by the shoot fly. However, when shoot flies are abundant (during the rainy season under moderate temperatures and high humidity) older plants may be attacked, but they do not produce the dead-heart symptoms. Instead, the damaged leaf becomes thin and papery, and wraps around the other leaves. As a result, the plants may fail to grow normally. Late infestations may also damage the panicle in the formative stage, resulting in rotting or drying up of a portion of the panicle affected by shoot fly damage. | 
Shoot fly
Shoot fly (Atherigona soccata) The adults are dark brown, and similar to a housefly, but nearly half the size. © Courtesy EcoPort (http://www.ecoport.org): Georg Goergen |
|
What to do:
|
|
African armyworm (Spodoptera exempta) It is an occasional but seriously destructive pest. It causes serious damage to young plants in years of armyworm outbreaks. The leaves are eaten away often leaving only the base of the stem. | 
African bollworm
Fully-grown caterpillar of the African bollworm (Helicoverpa armigera) is 3 - 4cm long. © A.M. Varela, icipe |
|
What to do:
|
|
African bollworm (Helicoverpa armigera) It causes damage to sorghum by eating the seeds. Damage is particular serious on compact headed sorghums. The caterpillars appear on the ear heads when the grains are in the milkripe stage. These caterpillars also feed on whorl leaves. A heavy infestation can cause an almost complete loss of yield. | 
African bollworm
Fully-grown caterpillar of the African bollworm (Helicoverpa armigera) is 3 - 4cm long. © A.M. Varela, icipe |
|
What to do:
|
|
Head bugs (Calocoris angustatus and Eurystylus oldi) Nymphs and adults of the head bug (Calocoris angustatus) and the African head bug (Eurystylus oldi) feed on developing kernels as panicles emerge from the boot. Head bugs are small (3 to 5 mm long, and about 1 mm wide) and variable in colour from yellowish green (C. angustatus), or pale brownish-yellow to dark brown with red markings (E. oldi). Females insert long, cigar-shaped eggs between the glumes or anthers of sorghum florets. Eggs usually hatch in less than a week. Nymphs and adults suck juice from developing kernels as panicles emerge from the boot. Kernels attacked early in development are shrivelled, small, and off-coloured, resulting in yield loss. Bug-damaged kernels become infected by secondary pathogens that further deteriorate grain quality. Feeding punctures are visible on older kernels. The life cycle is completed in about three weeks. At least two generations feed on the same crop when panicles in the field do not mature at the same time. |
|
|
What to do:
|
|
Other bugs A number of bugs feed on the milkripe sorghum grains: shield bugs including stink bugs (Nezara viridula, Acrosternum spp), Mirperus jaculus, Riptortus dentipes, Lygus bugs, blue bug (Calidea degrii) among others. The bugs puncture the seeds and suck the contents. Feeding punctures remain as dark spots on the testa. The seed weight is reduced; the rate of germination may be depressed. Sorghum is most susceptible to bug damage during the milk and soft dough stage. Injury normally is not damaging from hard dough to maturity. The damage is only of economic importance when bugs are present in large numbers. | 
Green stink bug
Green stink bug (Nezara viridula) (nymphs and adults). Adults are about 1.2cm long. (Host: Pearl Millet) © Russ Ottens, University of Georgia, Bugwood.org |
|
What to do:
|
- AIC (2002). Field Crops Technical Handbook.
- Acland J.D (1980). East African Crops. An Introduction to the Production of Field and Plantation Crops in Kenya, Tanzania and Uganda. FAO/Lonngman. ISBN: 0 582 60301 3.
- Anthony Youdeowei (2002). Integrated Pest Management Practices for the Production of Cereals and Pulses. Integrated Pest Management Extension Guide 2. Ministry of Food and Agriculture (MOFA) Plant Protection and Regulatory Directorate (PPRSD), Ghana, with German Development Cooperation (GTZ). ISBN: 9988 0 1086 9.
- Bohlen, E. (1973). Crop pests in Tanzania and their control. Federal Agency for Economic Cooperation (bfe). Verlag Paul Parey. ISBN: 3-489-64826-9.
- Buntin, G.D. (2005). Sorghum Insect Pests and Their Management. Cooperative Extension Service. The University of Georgia College of Agricultural and Environmental Sciences). Bulletin 1283/July, 2005.
- CABI. (2005). Crop Protection Compendium, 2005 Edition. © CAB International Publishing. Wallingford, UK. www.cabi.org
- Chauhan, R.S. and Palta, R.K (2004). Efficacy of insecticides and a neem derivative for control of sorghum midge Cantarinia sorghicola Coquilett. Indian Journal of Entomology Vol.66 (1): 88-89
- Handbook of crop protection recommendations in Ghana: An IPM approach Vol:1 Cereals and pulses. E. Blay, A. R. Cudjoe, and M. Braun (Editors). May 2000. Plant Protection & Regulatory Services Directorate and Integrated Crop Protection Project (ICP) German Development Co-operation (GTZ/PPRSD).
- Hill, D. (1983). Agricultural insect pests of the tropics and their control. 2nd Edition. Cambridge University Press. ISBN: 0-521-24638-5.
- ICRISAT. Sorghum genetic enhancement. Research process, dissemination and impacts. www.icrisat.cgiar.org
- KARI update: "Sorghum helps provide better food security", July 1997
- Lost Crops of Africa: Volume I: Grains (1996). NATIONAL RESEARCH COUNCIL Division on Policy and Global Affairs Development, Security, and Cooperation. Free online-version available: National Academies Press: www.nap.edu
- Nutrition Data www.nutritiondata.com.
- OISAT: Organisation for the Non-Chemical Pest Management in the Tropics. www.oisat.org
- Teetes, G. L. and Pendleton, B.B. Insect pests of sorghum. www.sorghumipm.tamu.edu
- Texas Agricultural Extension Service publication B-6004 (1996): Disease Response of Grain Sorghum Hybrids: Downy Mildew, Head Smut, Maize Dwarf Mosaic, and Anthracnose Texlab/Grains/Sorghum
- Tuleen DM, Frederiksen RA, Vudhivanich P, (1980). Cultural practices and the incidence of sorghum downy mildew in grain sorghum. Phytopathology, 70(9):905-908
- University of Nebraska (1985). Diseases Of Grain Sorghum www.nu-distance.unl.edu
- van den Berg, J., Obilana, A. B., Mgonja, M., Bronkhorst, L. (2005). Resistance of sorghum varieties to the shoot fly, Atherigona soccata Rondani (Diptera: Muscidae) in Southern Africa. International Journal of Pest Management, Vol. 51 (No. 1) 1-5. www.cababstractsplus.org
 Back
Back
