
|
Groundnut
Scientific name:
Arachis hypogaea
Order/Family:
Fabales: Fabaceae
Local names:
Njugu (Swahili)
Common names:
Peanut, earth nuts, monkey nuts
Pests and Diseases:
Aphids
Aspergillus crown rot
Bacterial wilt
Broomrape
Damping-off diseases
Groundnut blight
Groundnut hopper
Groundnut rosette disease
Leaf spots
Leafmining caterpillars
Milipedes
Root-knot nematodes
Rust
Sedges
Snails (Giant East African Snail)
Spider mites
Storage pests
Termites
Thrips
White grubs
|
Aphids (Aphis craccivora)
It is a serious pest as a vector of virus diseases, such as the rosette virus disease, a major constraint to groundnut production, particularly in the dry season. The groundnut aphid is black or dark brown in colour, variable in size (1.5 to 2.0 mm long) with two black cornicles (horns at the rear of the body), and a black tail.
- Early planting and dense close spacing are effective cultural practices.
Early planting allows plants to start flowering before aphids appear.
Dense planting provides a barrier to aphids penetrating in from field edges, discourages population build-up of aphids and reduces incidence of "rosette" disease. - Monitor and observe build-up of aphids and of natural enemies.
- Conserve natural enemies. Ladybird beetles are reported as important natural enemies in groundnuts.
- Use neem seed or leaf extracts if necessary.
- Do not cultivate groundnut or other legumes continuously on the same ground.
- Use tolerant or resistent varieties. The groundnut variety "Nyanda" is reported to be tolerant to aphids.

© James Litsinger. Reproduced from the Crop Protection Compendium, 2004 Edition. © CAB International, Wallingford, UK, 2004
Aspergillus crown rot (Aspergillus niger)
The fungus causes both seed and seedling rot and drastically reduces plant stand. In moist soil, seeds may be attacked and killed due to rotting. Seeds removed from soil show black sooty cover. The infected areas of seedlings are covered with black fungal spores. Mature plants are also attacked. Symptoms include permanent wilt of branches, and or wilting of entire plant. The dead and dried branches are easily detached from the collar region. Infected pods reveal patches of black sooty spores. Related species (Aspergillus flavus) causes deterioration of seeds.
It also produces the toxin (aflatoxin) in infected seeds that can cause death or other symptoms of toxicity when eaten by animals or humans.
A. flavus as a mould contaminant and toxin producer is much less serious during growth of the crop than during subsequent storage of kernels. Minimising moisture stress during growth can reduce invasion and toxin production by A. flavus.
- Rapid drying to moisture content of about 10% is the only means of preventing infection by A. flavus.
- Minimise damage to the nuts during harvesting because the fungus can easily enter a broken shell.
- Remove diseased crop debris from the field to reduce source of infection.

© Courtesy EcoPort (http://www.ecoport.org): Jürgen Kranz
Bacterial wilt (Ralstonia solanacearum)
It can cause serious losses, if a crop is infected early. Infected plants show water stress symptoms and may wilt suddenly without yellowing of the foliage, particularly, when temperatures are high.
- Rotate with cereals

© A.M. Varela, icipe
Damping-off diseases (iPythium spp., Rhizoctonia solani)
Damping-off diseases affect the initial establishment of a crop. Their main features include poor emergence and death of seedlings leading to poor stands in seedbeds and fields. Seeds may rot before germination. Affected seedlings that have emerged from the soil show water-soaking, browning and shrivelling of the stem at the soil level. They eventually fall over and die. Damping-off diseases are favoured by excessive wetness of the soil and low soil temperatures.
- Use certified disease-free seeds
- Avoid over-irrigation and excessive fertilisation with nitrogen fertilisers.
- Avoid fields previously planted with cotton or other related crops.

© Clemson University - USDA Cooperative Extension Slide Series, www.insectimages.org (Courtesy of EcoPort, www.ecoport.org)
Aspergillus crown rot (Aspergillus niger)
Aspergillus niger
The fungus causes both seed and seedling rot and drastically reduces plant stand. In moist soil, seeds may be attacked and killed due to rotting. Seeds removed from soil show black sooty cover. The infected areas of seedlings are covered with black fungal spores. Mature plants are also attacked. Symptoms include wilt of branches permanently, and or wilting of entire plant. The dead and dried branches are easily detached from the collar region. Infected pods reveal patches of black sooty spores. Related species (Aspergillus flavus) causes deterioration of seeds.
It also produces the toxin (aflatoxin) in infected seeds that can cause death or other symptoms of toxicity when eaten by animals or humans.
A. flavus as a mould contaminant and toxin producer is much less serious during growth of the crop than during subsequent storage of kernels. Minimising moisture stress during growth can reduce invasion and toxin production by A. flavus.
- Rapid drying to moisture content of about 10% is the only means of preventing infection by A. flavus.
- Minimise damage to the nuts during harvesting because the fungus can easily enter a broken shell.
- Remove diseased crop debris from the field to reduce source of infection.

© Clemson University - USDA Cooperative Extension Slide Series, Bugwood.org
Groundnut hopper (Hilda patruelis)
It is about 5mm in length, brown or green in colour with white marks and strips on the wings. The nymphs resemble the adults but without fully developed wings. These insects live in clusters or colonies, and are attended by ants that feed the honeydew excreted by the hoppers. These sucking insects attack the plants at the base of the stem, usually below ground level. The toxic saliva injected while feeding causes the plant to wither, turn yellow and die. The extent of damage can be important when the insect occurs in large numbers. The first sign of infestation is the presence of black ants.
- Use tolerant or resistant varieties. The groundnut variety "Nyanda" is reported to be tolerant to aphids and to the groundnut hopper (IAN, 2003).
Groundnut rosette disease
It consists of three types namely groundnut chlorotic rosette, groundnut green rosette and groundnut mosaic.
The disease is caused by a complex of different strains of groundnut rosette umbravirus. Symptoms vary depending on strain(s) present. They include yellowing, mottling and mosaic symptoms on leaves and stunting and distortion of the shoots. Older leaves are dark green, reduced in size, and show downward rolling of leaflet margins. If the plants are infected when they are young, they may not produce nuts.
The virus is transmitted by aphids (Aphis craccivora and A. gossypii), which feed on the undersides of the leaves.
- Sow early in the rains and plant close (high density planting).
- Plant tolerant / resistant varieties, e.g. "Asirya Mwitunde" .
- Remove virus-infected plants after harvest, and volunteer plants that are primary source of infection.
Leaf spots (Cercospora spp.)
Symptoms of early leaf spot (Mycosphaerella arachidis, Cercospora arachidicola) consist of sub-circular dark brown spots produced on the upper leaflet surface. The spots are of lighter shade of brown on the lower side of the leaflets. Yellow halo is seen around the brown spots. Oval to elongate spots are also seen on stems, petioles, and pegs. Late leaf spot can be distinguished from those of early leaf spot.
Late leaf spots (M. berkeleyi, Cercosporidium personatum) are darker with no or light yellow halo. The late leaf spots on the lower leaflet surface are rough in appearance. They exhibit circular rings of fungus fruiting structures on the lower leaflet surface. Severe disease attack leads to shedding of leaflets resulting in premature ageing of the crop. Oval to elongate spots similar to early leaf spot are also formed on stems and pegs. Late leaf spot attack is usually seen along with rust disease.
- Plant tolerant / resistant varieties, if available.
- Collect and destroy the infected crop debris.
- Follow cereal-cereal- groundnut crop rotation.
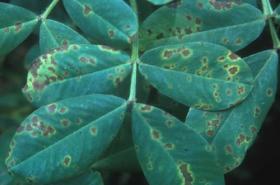
© Courtesy EcoPort (http://www.ecoport.org): Jürgen Kranz
The groundnut leafminer (Aproaerema modicella)
It is a common pest of groundnuts in South and South-East Asia and a major pest in India, and it has recently invaded Africa. It was first found in Uganda in 1998 and is now recorded in Mozambique, Malawi, Democratic Republic of Congo and South Africa. In all African countries where this leafminer has been found, the pest has reached epidemic densities and severe yield losses have been observed on groundnut (The New Vision. 2004; Kenis and Cugala, 2006). The adult is a mottled moth, with a full wing span of up to 18mm. The moth lays eggs on the underside of the groundnut leaf and petioles. Yellowish green caterpillars hatch, tunnel into the leaves and feed between the upper and lower epidermis of the leaf. Mined leaves become distorted within a few days. Caterpillars are grey-green with a shiny black head. There are five larval instars. The first instar has an average length of 0.56 mm. At pupation, they rarely exceed 8 mm in length.
Three or four mines per groundnut leaflet can cause so much distortion that a leaf exposes as little as 30% of the potential photosynthetic area to the sun. Later, when the caterpillar becomes too large to occupy the mine, they emerge to the leaf surface and either fold over a single leaf and hold it down with silk, or web together two or more leaflets. They live and feed in the shelter they have constructed. Pupation takes place inside the webbed leaflets. Damaged leaves become brownish, rolled and dry, which results in early defoliation and affects the growth and yield of the plants.
- Use tolerant/resistant varieties. In Uganda, it has been reported that the variety "Egola-1" had shown signs of relative resistance.
- Plant during the first short rains when normally the miner population is low.
- Avoid drought stress by irrigating or early sowing so as to avoid periods when drought is likely. Plants that are drought stressed are much more susceptible to leafminer attack than irrigated plants.
Millipedes (Peridontopyge spp.)
Millipedes are among the economically important soil pest of groundnuts. They are brown to blackish in colour and curl when disturbed. They attack groundnut seedlings, between planting and approximately 20 days after planting, feeding on the emerging cotyledons and moving to the root system at the collar region. The cortex is often damaged serving as an entry point for secondary infection by microorganisms. The development of plants surviving the attack is often retarded.
Millipedes also attack maturing groundnut during pod formation, i.e. when the pods are still soft. Immature pods from severed pegs are often perforated and thus suffer secondary infection or invasion by rot-causing organisms such as Aspergillus flavus. Millipedes may also damage flowers. Birds are main predators of millipedes.
- Practise good sanitation.
- Prepare land properly.
- Select sites away from forest (breeding sites for millipedes).
- Cover exposed pods.
- Close cracks in the soil.
- Use varieties with pods well buried.

© Courtesy EcoPort (http://www.ecoport.org) : Agricultural Research Council of South Africa
Root-knot nematodes
They are widespread but their seriousness in yield reduction is unknown. They cause plant stunting, gall or knot formation on roots, and in severe cases wilting of affected plants
- Rotate with cereals

© A. M. Varela, icipe
Rust (Puccinia arachidis)
Pustules (spots or blisters) can form on all aerial plant parts except flowers. Orange coloured pustules first appear on the lower surface of leaflets. Later, pustules may appear on the upper surface of the leaflets. The pustules on the stem are elongate and elevated. The pustules when mature rupture to release masses of reddish-brown spores, which blown by wind, spread the disease from plant to plant and far away to other groundnut fields.
- Plant resistant varieties if available.
- Remove volunteer groundnut plants from the field to check build-up of rust infection.
- Adopt cereal-cereal-groundnut crop rotation.
- Adjust the sowing time to avoid the most conducive environmental conditions for rust development i.e., high humidity, cloudy weather.

© A. M. Varela, icipe
Storage pests: moths and beetles
Stored groundnuts are attacked by moths (Ephestia cautella, Plodia interpunctella, Cadra cautella), and beetles (Caryedon serratus, Tribolium castaneum, Trogoderma granarium).
The larvae of moths and the grubs and adult beetles bore into and damage seeds. Moths cause extensive webbing. The bruchid beetle Caryedon serratus is the major pest of groundnut in pod shell in West Africa. A good post harvest pest management programme based on good storage practices is very important.
- As most post-harvest groundnut pests except bruchids are unable to penetrate intact pods, leaving the crop in the shell for as long as possible during storage is an effective method of limiting damage.
- Research into low cost technology to protect stored groundnut showed that Samadaka (Swartzia madagascariensis), 2 kg of powdered fruits to treat 100 kg groundnuts, was very effective against bruchids and moths for the groundnuts stored in granaries.
- Addition of sand as an abrasive material at the farm level was very effective (INPhO Compendium)

© Clemson University - USDA Cooperative Extension Slide Series, Bugwood.org\n\n \n
Termites
Termites are serious groundnut pests throughout the southern African region and West Africa. Species of Microtermes and Odontotermes are the most damaging, while Macrotermes cause occasional damage. The small-sized Microtermes spp., in particular, attack and invade growing groundnut plants through the roots and stem near ground level, hollowing them out and causing the plants to wilt and die with a consequent reduction in crop stand.
Roots damaged by other soil pests, such as white grubs, are also prone to attack by termites. Some termite species (Macrotermes spp., Hodotermes mossambicus) cut off stem bases, and may cause 25-100% of plant losses. As the crop ripens the outer layers of the pods are scarified (removal of soft corky tissue between the veins of the pod) by termites allowing contamination of the seed with soil fungi, such as Aspergillus flavus, which produce lethal "aflatoxins".
Scarification of pods is by far the most common type of termite damage at plant maturity, a factor often aggravated by late harvest. Scarification as high as 30% has been reported. Infested plants are not obviously diseased and are frequently harvested and contaminate the rest of the crop. Species such as Microtermes spp. also penetrate the pod to feed off the soft inner lining, filling the pod with soil. This form of attack leads to additional loss through premature germination of kernels. Stacks of plants left drying in the fields are also frequently attacked by species such as Odontotermes spp. with farmers losing between 30-40% of their crop at this stage.
Termite damage is generally most serious towards the end of the growing season just prior to harvesting, and it is particularly serious during periods of drought (ARC/LRN. 2007).
- Remove residues of previous cereal crops (sorghum, millet and maize). Plant residues left in the field serve as food for termites, which may infest the new crop. Termite infestation of 100% has been observed in groundnut crops with high plant residues.
- Planting should be carried out early enough to avoid drought periods. Moisture deficiency may stress a crop and lead to attack by termites due to low vigour.
- Harvest promptly. Research has shown that termite damage increases with delay in harvest. Furthermore, most groundnut-producing areas in sub-Saharan Africa experience drought and high temperatures during the later part of the growing season, conditions that favour termite infestation as well as fungus (A. flavus) infection of pods leading to aflatoxin formation in seeds.
- The complete destruction of mounds and removal of queen termites are effective control measures against mound-building species (Macrotermes spp.). Partial destruction of mounds is unlikely to solve the problem, since replacement reproduction may develop from the remaining termites.
- It has been reported that close spacing in groundnut helps to deter termite infestation, although the reason for this was not given. However, high density sowing, followed by thinning of surviving plants where necessary to reduce competition, offsets anticipated losses due to termites.

© A. M. Varela, icipe
Thrips (Megalurothrips sjostedti and Frankliniella schultzei)
Several species of thrips attack groundnuts. They have been reported as important pests of groundnuts in Uganda. The flower thrips (Frankliniella schultzei and Megalurothrips sjostedti) infest mainly buds and flowers. Attacked flowers are discoloured and scarred; terminal leaf buds are blackened and distorted after unfolding. Other species of thrips (e.g. Scirtothrips dorsalis and Caliothrips indicus) infest foliage.
Thrips feeding causes yellowish-green patches on the upper leaf surface and brown necrotic areas and silvery sheen on the lower surface of the leaf; leaves become thickened and some curling occurs. In severe infestations, young leaves are severely deformed, plants are stunted and leaves are blighted.
- Conserve natural enemies. Thrips are attacked by predatory thrips, lacewings and predatory bugs.
- Whenever necessary spray the crop with botanicals, such as plant extracts (e.g. garlic, rotenone, neem, pyrethrum, etc.). A mixture of garlic and pepper has been recommended for organic growers in USA.
- Plough and harrow before transplanting. This can be useful in reducing thrips attacks by killing pupae in the soil.

© Steve L. Brown, University of Georgia, Bugwood.org
White grubs (Schyzonycha spp.)
Whitegrubs are the larvae of scarab "chafer" beetles. They are white, C-shaped with a brown head and three pairs of legs. Many species of white grubs are associated with groundnut damage in parts of sub-Saharan Africa. The most important are Schyzonycha species.
White grubs attack plants at all stages of growth. They eat roots and damage pods of groundnuts. White grubs feed mainly on the taproots and/or peripheral roots leading to stunting or death. They inflict cuts in the crown region of taproots; these lesions are often invaded by rot-causing fungi. White grubs also cut out pods from the base of groundnut pegs and destroy larger, soft pods. Plants are often attacked in a row. White grubs seem to prefer soils with sandy or loamy sand textures and are seldom observed in clay soils.
- Allow enough time between manure application and planting of groundnut. The excessive use of organic manure in groundnut farms has been observed to increase the incidence of white grubs, especially when manure is applied during the cropping season.
- Deep ploughing or hand hoe tillage exposes soil pests to desiccation and to predators, thus helping to reduce their numbers and damage.

© A. M. Varela, icipe
| General Information and Agronomic Aspects | Information on Diseases | |||
| Information on Pests | Information Source Links |
 |
| Geographical Distribution of Groundnut in Africa |
Groundnuts are a small erect or trailing herbaceous legume, about 15 to 60 cm high. The fruit is a pod with one to five seeds that develops underground within a needle-like structure called a peg. The seeds are rich in oil (38-50%), protein, calcium, potassium, phosphorus, magnesium and vitamins. Groundnuts have also considerable medicinal value. They are reported to be useful in the treatment of disease such as haemophilia, stomatitis, and diarrhoea.
Most of the world production of groundnuts is crushed for oil that is used mainly for cooking. The press cake from oil extraction is a feed rich in protein but is also used to produce groundnut flour, which is used in many human foods. The seeds or kernels are eaten raw, boiled or roasted, made into confectionery and snack foods, and are used in soups or made into sauces to use on meat and rice dishes. The vegetative residues from the crop are excellent forage.
In sub-Saharan Africa, groundnuts are a basic staple crop, cultivated mainly by small-scale farmers both as subsistence and as a cash crop. It is an important source of protein and other nutrients for poor rural communities. In Africa, groundnut yields are traditionally low, due to unreliable rains, little technology available to small-scale farmers, pest and disease occurrence, poor seed variety, and increased cultivation on marginal land (ICRISAT).
Nutritive Value per 100 g of edible Portion
| Raw or Cooked Peanuts | Food Energy (Calories / %Daily Value*) | Carbohydrates (g / %DV) | Fat (g / %DV) | Protein (g / %DV) | Calcium (g / %DV) | Phosphorus (mg / %DV) | Iron (mg / %DV) | Potassium (mg / %DV) | Vitamin A (I.U) | Vitamin C (I.U) | Vitamin B 6 (I.U) | Vitamin B 12 (I.U) | Thiamine (mg / %DV) | Riboflavin (mg / %DV) | Ash (g / %DV) |
| Peanut Oil | 884.0 / 44% | 0.0 / 0% | 100.0 / 154% | 0.0 / 0% | 0.0 / 0% | 0.0 / 0% | 0.0 / 0% | 0.0 / 0% | 0.0 IU / 0% | 0.0 / 0% | 0.0 / 0% | 0.0 / 0% | 0.0 / 0% | 0.0 / 0% | 0.0 |
| Peanuts raw | 567 / 28% | 16.1 / 5% | 49.2 / 76% | 25.8 / 52% | 92.0 / 9% | 376 / 38% | 4.6 / 25% | 705 / 20% | 0.0 IU / 0% | 0.0 / 0% | 0.3 / 17% | 0.0 / 0% | 0.6 / 43% | 0.1 / 8% | 2.3 |
| Peanuts dry-roasted | 585 / 29% | 21.5 / 7% | 49.7 / 76% | 23.7 / 47% | 54.0 / 5% | 358 / 36% | 2.3 / 13% | 658 / 19% | 0.0 IU / 0% | 0.0 / 0% | 0.3 / 13% | 0.0 / 0% | 0.4 / 29% | 0.1 / 6% | 3.6 |
Because pods develop underground and must be recovered at harvest, crumbly, well-drained soils are preferred, but plants grow and develop adequately on heavier clay soils. For optimum growth, soil pH should be in the range 5.5 to 6.5, though Spanish types tolerate more acid conditions (pH 4.5) and some cultivars grow well in alkaline soils up to pH 8.5.
After ploughing and harrowing to a fairly good tilth, ridges, which are 80 cm apart with flattish tops, should be made so that two rows of nuts can be planted on each ridge. Seeds for planting should be well selected: they should be clean, well filled and without any blemishes. Seeds for planting should be kept in their pods and shelled a few days before planting. Planting depth is like maize about 5 to 8 cm. Seed rate is 40 to 50 kg/ha depending on the size of the seeds.
There are 2 types of groundnuts:
- Bunch type
- Runner type
Bunch varieties such as Red Valencia mature within 90 to 100 days, while runner types such as "Homa Bay" mature in 120 to 150 days (require a longer growing season).
| Variety | Mean kernel yield Kg/ha |
| "Red Valencia" | 1500 |
| "Severe 116" (white) | 1250 |
| "Texas Peanut" | 1360 |
| "Bukene" | 1530 |
| "Manipintar" | 2450 |
| "Makulu Red" | 2720 |
| "Altika" | 900 |
| "Homa Bay" | 770 |
| "Asirya Mwitunde" | 1300 |
With good husbandry current farmers' yields of between 450-700 kg/ha could be doubled.
There is an increase in the yield of groundnuts when intercropped with early maturing pigeon pea.
The only peculiar nutrient requirement is for calcium (Ca) in the podding zone. Calcium is absorbed directly by the pods, if soil moisture is adequate. A shortage of Ca in that zone will result in empty pods (especially in Virginia cultivars). The crop's needs for nitrogen should be satisfied with symbiotic fixation by strains of Rhizobium of the cowpea group, so nitrogen fertilisers are not generally required. In some areas of acid soils, lime is applied to raise the pH and supply Ca. Moisture stress during flowering or pod filling reduces yield so that irrigation during those periods to minimise or eliminate the stress increases production and seed quality. Where yields are unsatisfactory (heavily eroded soils) an application of 200 kg/ha of rock phosphate is recommended.
Dig a few plants up to see if the nuts are ready. The nuts should be brown on the outside, firm and dry. Usually at maturity the inside of the pods is grey and some rattling occurs when pods are shaken. Severe disease of foliage sometimes results in harvesting before seeds are fully mature. Plants should be carefully dug out to avoid nuts breaking off and remaining in the ground. Dry for 2-3 days, then rip the pods from the bushes and place them on mats to dry for another 7-10 days to about 10% moisture.
Shelling should be done by hand. Broken, dirty or damaged nuts should be discarded as these will lower the quality and hence the selling price. When thee groundnuts are poorly dried and stored, they pick mold and dirt, which attracts a fungi, that releases aflotoxin chemicals that are dangerous to human health especially the liver. Nuts to be used as seed the following year should not be shelled.
- AIC (2002). Field Crops Technical Handbook. Nairobi Kenya.
- ARC/LRN. (2007). Termites in crops.
- Blay, E., Cudjoe, A. R., Braun, M. (eds). (2000). Handbook of crop protection recommendations in Ghana: An IPM approach Vol:1 Cereals and pulses. May 2000. Plant Protection & Regulatory Services Directorate and Integrated Crop Protection Project (ICP) German Development Co-operation (GTZ/PPRSD).
- Bohlen, E. (1973). Crop pests in Tanzania and their control. Federal Agency for Economic Cooperation (bfe). Verlag Paul Parey. ISBN: 3-489-64826-9.
- Brunt, A.A., Crabtree, K., Dallwitz, M.J., Gibbs, A.J., Watson, L. and Zurcher, E.J. (eds.) (1996 onwards). Plant Viruses Online: Descriptions and Lists from the VIDE Database. Version: 20th August 1996.
- CAB International (2005). Crop Protection Compendium, 2005 edition. Wallingford, UK www.cabi.org
- Groundnut (Arachis hypogaea), women and development: Impact on nutrition and women's role in Western Africa. www.forest.mtu.edu
- ICRISAT, www.icrisat.org
- INPhO. Post-harvest Compendium. Groundnut. www.fao.org
- IPM CRSP. Seventh Annual Report. Overview of the African Site in Uganda. www.oired.vt.edu
- International Arachis Newsletter (IAN). No. 23, 2003.
- J.D. Acland (1980). East African Crops. FAO/Longman. ISBN: 0 582 60301 3
- Kenis, M., Cugala, D. (2006). Prospects for the biological control of the groundnut leaf miner, Aproaerema modicella, in Africa. CAB Reviews: Perspectives in Agriculture, Veterinary Science, Nutrition and Natural Resources, 1, No. 31, 9 pp. Insect Sci. Applic. Vol. 21, No. 3 pp. 257-265. www.pestscience.com
- Nutrition Data www.nutritiondata.com.
- OISAT: Organisation for Non-Chemical Pest Management in the Tropics. www.oisat.org
- Participatory evaluation of the distribution, status and management of the groundnut leafminers in the Teso and Lango farming systems. Final technical report. NARO/DFIFD COARD Project. October 2004. By George Epieru Naro Saari).
- Paulraj, M.P. & S. Ignacimuthu (2006). Integrated control of groundnut leafminer. Entomology Research Institute Loyola College, Chennai. www.thehindu.com
- The New Vision. Uganda's leading website. Poorly processed peanut butter causes cancer http://www.newvision.co.ug
- Umeh, V. C. , Walyyar, F. , Traoré, S., Chaibou, I. M., Omar, B., Detognon J. (2001). Farmers' opinions and influence of cultural practices on soil pest damage to groundnut in West Africa. Mini Reviewwww.bioline.org.br
- Umeh, V. C., Youm, O., Waliyar, F. (2001). Soil Pests of Groundnut in sub-Saharan Africa - A Review. Insect Sci. Applic. Vol. 21, No. 1, pp. 23-32. Bioline International, 1989 - 2007, Site last up-dated on 14-May-2007. www.bioline.org.br
- Youdeowei, A. (2002). Integrated Pest Management Practices for the Production of Cereals and Pulses. Integrated Pest Management Extension Guide 2. Ministry of Food and Agriculture (MOFA) Plant Protection and Regulatory Services Directorate (PPRSD), Ghana, and German Development Cooperation (GTZ). ISBN: 9988 0 1086 9.
 Back
Back
