
|
Bacterial wilt
Scientific name:
Ralstonia solanacearum (Pseudomonas solanacearum)
Order/Family:
Burkholderiales: Ralstoniaceae
Type:
disease (bacterial)
Common names:
bacterial wilt, brown rot, moko disease (banana), slime disease (potato), southern bacterial blight (tomato), seedling rot
Host plants: African Nightshade
Bananas
Eggplant
Groundnut
Peppers
Potato
Tomato
Tobacco
|
| General Information on Disease and Damage | Cultural practices | |||
| Biology and Ecology of Bacterial Wilt | Information Source Links | |||
| Pest and disease Management |
General Information on Disease and Damage
Geographical distribution
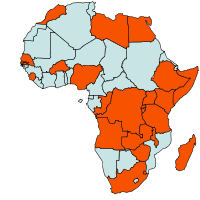 |
| Geographical Distribution of Bacterial wilt in Africa (red marked) |
Introduction
Bacterial wilt is widespread in tropical, subtropical and warm temperate areas throughout the world. Its occurrence has now also been reported from temperate zones. In Africa it has been reported in Angola, Burkina Faso, Burundi, Congo, Ethiopia, Gabon, Gambia, Kenya, Madagascar, Malawi, Mauritius, Mozambique, Nigeria, Réunion, Rwanda, Senegal, Seychelles, Sierra Leone, Somalia, South Africa, Swaziland, Tanzania, Tunisia, Uganda, Zaire, Zambia and Zimbabwe (CABI, 2005).
Damage
R. solanacearum constitutes a serious obstacle to the cultivation of many solanaceous plants in both tropical and temperate regions. The greatest economic damage has been reported on potatoes, tobacco and tomatoes. It can sometimes cause total crop losses. Disease severity mostly increases if R. solanacearum is found in association with root nematodes. In tobacco, nematode infestation changes the physiology of the plants, causing susceptibility to bacterial wilt. Experiments in India showed that the combined pathogenic effects of R. solanacearum and root-knot nematodes (Meloidogyne javanica) were greater than the independent effects of either (CABI, 2005)
The bacterium is a quarantine organism. The occurrence of different races and strains of the pathogen with varying virulence under different environmental conditions presents a serious danger to European and Mediterranean potato and tomato production. Absence of the bacterium is an important consideration for countries exporting seed potatoes.
The bacterium is a quarantine organism. The occurrence of different races and strains of the pathogen with varying virulence under different environmental conditions presents a serious danger to European and Mediterranean potato and tomato production. Absence of the bacterium is an important consideration for countries exporting seed potatoes.
Host range
Primary hosts: Lycopersicon esculentum (tomato), Nicotiana tabacum (tobacco), Solanum melongena (aubergine), Solanum tuberosum (potato), Musa (banana), Musa paradisiaca (plantain) and Heliconia.
Secondary hosts: Anthurium, Ricinus communis (castor bean), Zingiber officinale (ginger), Arachis hypogaea (groundnut), Capsicum annuum (bell pepper), Colocasia esculenta (taro), Curcuma longa (turmeric), Gossypium (cotton), Hevea brasiliensis (rubber), Ipomoea batatas (sweet potato), Manihot esculenta (cassava).
Wild hosts: Solanum nigrum (black nightshade), Galinsoga parviflora (gallant soldier), Portulaca oleracea (pussley), Urtica dioica (stinging nettle).
Symptoms
The disease causes rapid wilting and death of the entire plant without any yellowing or spotting of leaves. All branches wilt at about the same time. When the stem of a wilted plant is cut across, the pith has a darkened, water-soaked appearance. There is a greyish slimy ooze on pressing the stem. In later stages of the disease, decay of the pith may cause extensive hollowing of the stem.
Bacterial wilt causes no spotting of the fruits. Affected roots decay, becoming dark brown to black in colour. If the soil is moist, diseased roots become soft and slimy.  |
| Water test for detection of bacterial wilt. Note bacterial strands oozing from infected tissue. |
| © A. M. Varela and A. A. Seif, icipe |
| Bacterial wilt of potato:
The infested leaves wilt during the (sunny) day and sometimes recover during cool hours. The wilting is similar to the result of lack of water. During the rapid development of the disease, the entire plant wilts quickly without yellowing. Other symptoms could be wilting of only a part of the stem, or one side of the leaf/stem. The stem wilts or dries up completely and the remainder of the plant remains healthy. When the diseased tuber is cut, it shows a browning of the vascular ring and the immediate surrounding tissues On the cut surface, a creamy fluid usually appears on the vascular ring. |
|
Moko disease of banana: Initially one of the youngest three leaves turns pale-green or yellow in colour and breaks down at the petiole and the pseudostem. Later all the other leaves collapse around the pseudostem. An infected finger or fruit shows dry and rotted pulp that is coloured brown or black, and the presence of bacterial discharges.
Affected plant stages
Vegetative growing stage. Affected plant parts
Leaves, roots, seeds, fruits, stems, vegetative organs and whole plant. Symptoms by affected plant part
Growing points: wilting. Leaves: wilting.
Roots: rot.
Stems: internal discoloration; creamy exudates; wilt.
Vegetative organs: internal discoloration.
Whole plant: plant death; dwarfing.
Biology and Ecology of Bacterial Wilt
Source of infection and spread
The bacterium is soil-borne and can survive in soil for long periods. However, some soils are conducive to bacterial wilt and other suppressive. Important soil factors affecting the occurrence and persistence of the pathogen are soil moisture and antagonistic microorganisms. Soil type influences soil moisture and population of antagonistic microorganisms, which in turn affect the survival of R. solanacearum in soil.Bacterial wilt (R. solanacearum) has a very wide host range and infects all nightshade plants (members of Solanaceae). Weed hosts include black nightshade, lantana and Jimson weed. It infects plants through roots. As the roots of wilted plants decay, the bacteria are released back into the soil. Infestation by root-knot nematodes aggravates the disease. The bacterium is especially destructive in moist soils at temperatures above 24° C. It is sensitive to high pH (alkaline soils), low soil temperature, low soil moisture and low fertility levels. Spread is effected by running water, movement of infested soil and also diseased seedlings.
Bacterial wilt can also be spread on vegetative propagating material. Therefore, plant quarantine of potentially infested plant material is very important to avoid long-range dispersal.
(Hayward 1991)
Conditions that favour disease development
1. Crop residues infected with Ralstonia solanacearum left in the field . 2. Injured roots caused by farm tools or by soil pests
3. Warm temperature and high soil moisture
4. Slightly acidic soils
5. Poor and unfertile soil
6. Infestation by root-knot nematodes
Pest and disease Management
Pest and disease Management: General illustration of the concept of infonet-biovision
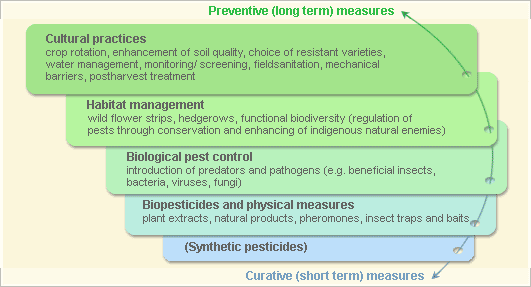
Further below you find concrete preventive and curative methods against Bacterial wilt.
Cultural practices
- Crop rotation is not effective as the pathogen can survive for a long period (several years) in the soil and also attack a wide range of crops and solanaceous weeds
- Use plant varieties that are tolerant / resistant to bacterial wilt. In Kenya, the following tomato varieties have been claimed to be resistant to bacterial wilt: "Fortune Maker", "Kentom", and "Taiwan F1".
- Do not grow crops in soil where bacterial wilt has occurred.
- Rogue out wilted plants from the field to reduce spread of the disease from plant to plant
- Control root-knot nematodes since they could facilitate infection and spread of bacterial wilt
- Where feasible, extended flooding (for at least 6 months) of the infested fields can reduce disease levels in the soil
- Soil amendments (organic manures) can suppress bacterial wilt pathogen in the soil
Disease avoidance
Since high soil temperatures and soil moisture enhance bacterial wilt development, damages can be minimized by changing the date of planting, considering seasons which are less favourable for disease development.Intercropping
In some developing countries intercropping has been used as a means of reducing pathogen populations in the soil and root-to-root transmission. In Burundi, growing potato with bean showed a lower incidence of bacterial wilt, indicating that a bean intercrop, which has a dense root system and grows quickly, was better than a crop such as maize, which develops slower and has a more dispersed root system.
(Hayward 1991)
Information Source Links
- Beije C.M, Kanyagia S.T., Muriuki S.J.N., Otieno E.A., Seif A. and Whittle A.M. (1984). Horticultural Crops Protection Handbook. National Horticultural Research Station. Thika, Kenya.
- CAB International (2005). Crop Protection Compendium, 2005 edition. Wallingford, UK www.cabi.org
- Dobson H., Cooper J., Manyangarirwa W., Karuma J. and Chiimba W. (2002). Integrated Vegetable Pest Management. Natural Resources Institute (UK) ISBN: 0-85954-536-9
- Hayward, A.C. (1991). Biology and Epidemiology of Bacterial Wilt caused by Pseudomonas Solanacearum. Annual Reviews Phytopathol. 29: 65-87.
- OISAT: Organisation for Non-Chemical Pest Management in the Tropics www.oisat.org
- Varela, A.M., Seif, A. and Löhr, B. (2003). A Guide to IPM in Tomato Production in Eastern and Southern Africa. ICIPE. ISBN: 92 9064 149 5
 Back
Back

