This Hepatitis Day, the 185 million people worldwide living with chronic Hepatitis C (HCV) and their loved ones ought to be celebrating a major medical accomplishment: the release of sofosbuvir, a new medication which cured 90% of HCV-infected patients in clinical trials. It is too soon to celebrate, however, as this life-saving drug remains financially out of reach for most of those affected.
Gilead Sciences, the manufacturer, has decided to sell the medication, named Sovaldi, at a cost of $84,000 per twelve-week treatment regimen. Raymond Schinazi, one of the scientists who helped formulate the drug, estimates that this same treatment regimen costs only $1,400 to produce. At this price, insurance companies and government programs will be reluctant to cover the medication, and it will be inaccessible for most people living in low- and middle-income countries, who account for 90% of HCV cases worldwide.

Gilead has justified the price by claiming that it reflects the real “cost per cure” over a patient’s lifetime, including treatment they would have to pay for if they hadn’t taken Sovaldi (things like long-term medication and liver transplants, which cost nearly $600,000). This allows the company to claim that the medicine is a money saver in spite of the shocking price tag. Other pharmaceutical allies also rationalize the cost by saying that this is simply the price of innovation, as drug companies spend anywhere from $4 to11 billion in research and development for a new medication. While this is a significant financial burden for these companies, Gilead would still have a high profit margin even if the medication was priced affordably; after all, they have 185 million potential consumers for the product. Common practices like ‘evergreening’ (by which pharmaceutical companies alter the composition of a product before the patent expires, thereby allowing them to maintain their patent and thus their profit margin) make it likely that Gilead will continue to reap huge profits for decades to come. Sovaldi has already broken profit records by earning $5.8 billion in its first two quarters, which makes it all the more difficult to believe that the cost to consumers is justifiable.
Already insurance companies in the United States have balked at the cost of treatment, and the costs could put unreasonable strain on Medicaid programs, prisons, and veteran’s services, which are examples of patient groups that most need the drug. Some insurers are refusing to cover Sovaldi at all, while Kaiser Permanente and other insurance agencies have decided to cover it only for the patients with advanced HCV, which will do little to slow disease progression in still-healthy patients.
The prohibitive cost has put health advocates in a bind. Alan Franciscus, of HCAdvocate, an organization that publishes an HCV-focused newsletter on www.hcvadvocate.org and runs 40-60 trainings each year with public health workers, patients and doctors, is one example. He has found that Gilead has had good patient assistance program for uninsured patients in the past, which he has encouraged people to use. Yet in spite of this he has remained concerned about how the cost will affect insurance companies’ willingness to cover the medications, and has felt that he has had to “be silent on the question of cost. There have been a lot of people talking about it, which has encouraged companies to deny treatment. That is the last thing I want to do, although the prices are exorbitant.” Unfortunately, he does not anticipate the cost of medications declining anytime soon, noting that “even as new drugs come along, the prices get higher. The first lines of treatment were in the $30,000 range, and now they have slowly inched up to the $100,000 range. When there is innovation pharmaceutical companies try to get the best price they can.”
Abroad, Gilead has already promised to offer a tiered pricing system, which will provide Sovaldi regimens for as low as $2,000 to low- and middle-income countries such as India, Indonesia, and Egypt. Despite this significant discount, however, the still hefty price tag will do little to help those most in need. Take Egypt, for example, where 10% of the population suffers from chronic HCV. In Egypt, 40% of laborers work in the informal economy and make wages of $165 per month; at that rate, it would take over a year to earn $2,000. Treating everyone infected with HCV in Egypt would cost 3,154 times the country’s total health spending in 2011.
Critics have likened the situation with sofosbuvir to the availability of HIV medications, with pharmaceutical companies withholding costly medications that have the capacity to dramatically slow the pace of the epidemic, a trend that was only reversed with a great deal of dedicated advocacy work and international collaboration. HCV was discovered shortly after HIV, but hepatitis has not shared the same publicity and concomitant funding, and has only recently become a topic of international public health discussion on a large scale. The reason? Alan of HCV Advocate notes that those diagnosed with HCV in the United States are typically particularly disadvantaged, “and the disease is so often associated with injection drug users, a population that is generally ignored and hated.”
What needs to happen in order to make sofosbuvir accessible to the millions of people who need it? Organizations such as UNITAID can step up to make funding for HCV medications a priority, and creation of a Medical Patents Pool for HCV (such as exists for HIV medications) would help build collective bargaining power internationally.
Yet these strategies should not be implemented in lieu of pressuring corporations and the US government to reduce the cost of medications at the source. While there have been congressional hearings about the cost of Sovaldi, Alan believes that national funding mechanisms are partially to blame, since government-funded research “has been minimal—it’s a drop in the bucket.” Government funding of drug research needs to be improved and increased, and people can get involved by supporting the excellent advocacy work being carried out by HCV Advocate and the National Viral Hepatitis Roundtable. Finally, we should continue to support harm reduction measures that prevent further spread of the disease, test the millions of people who are unaware of their status, and treat everyone infected, not only the severe cases.
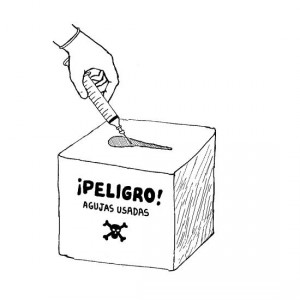
Prevention efforts like syringe exchanges and sharps collection can go a long way in reducing the spread of HCV