Top-down control

All organisms require energy and nutrients in some form to survive and grow. Thus, the existence and total biomassof ecosystems are ultimately regulated from the "bottom up", that is, by availability of resources such as sunlight, inorganic nutrients, water and other abiotic factors. Nevertheless, on more localized scales, the abundances of organisms at lower trophic levels can be strongly affected by their own predation, that is, by top-down control. Ecosystems often have been assumed to be controlled from the bottom:up, wherein nutrients and light (Sunlight) stimulate primary production by plants, and the greater primary production in turn supports more animal production at higher trophic levels. But this is not the whole story. A simple way to envision top-down control is as follows: because the predator eats prey, the prey population is reduced, so when predators are removed, the prey species can become more abundant. For example, evidence of such top-down control of prey populations was seen when large predators like wolves were extirpated from much of the US and deer became very abundant, indeed a pest, in many areas. Even in Yosemite National Park, reduced numbers of bobcats, cougars and coyotes led to increased numbers of mule deer, which then led to declines in evening primrose and young black oaks, which are grazed by deer.
Contents
Trophic cascades
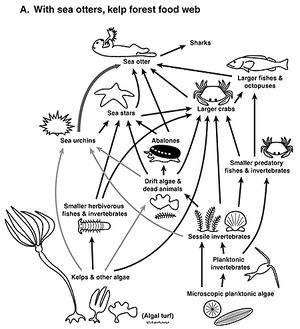
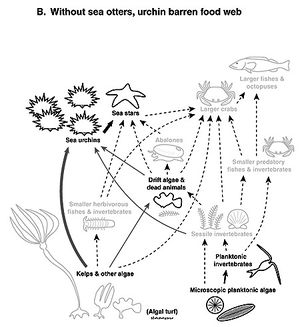
Some other clear examples of strong top-down control are seen in the marine environment. In the Pacific Coast of North America there are “kelp forests” of very large brown algae. These are grazed by sea urchins, which are, in turn, eaten by sea otters. When sea otter populations are reduced (as they were a century ago because of hunting, and again lately due to killer whale predation) the sea urchin populations can grow out of control and graze down most the kelp, creating “urchin barrens.” Unchecked by otters, they can bloom and basically destroy the kelp forest by eating huge amounts of kelp. When population control of prey by their predators (as in the sea otter/sea urchin interaction) then affects the next level down, the kelp, it is referred to as a “trophic cascade.” Trophic cascades are now known from a wide range of marine, freshwater, and terrestrial ecosystems.
Other systems have been affected by the loss of filter feeders such as oysters. It has been suggested that the populations of oysters in Chesapeake Bay used to be enough to filter all the water (including its phytoplankton residents) in the bay in about one week. Although this has since been considered an overestimate, it is clear that filter-feeding aimals can have strong impacts on water quality. as As a result of overfishing and several devastating diseases, the oyster population is currently only a tiny fraction of what it used to be, and algal blooms are more common, as the phytoplankton can no longer be controlled by the filter feeders (increased levels of nutrients exacerbate this effect). Top-down ecology is also seen in lakes, rocky shores, terrestrial, and many other ecosystems.
Top-down control and behavior: The ecology of fear

Top-down control of lower trophic levels as a result of direct consumption and reduction of prey population size is referred to as “density-mediated” control, because predators reduce density of prey. However, predators can affect populations of their prey without actually eating them but in a more indirect ways, for example by influencing their behavior, physiology, or morphology. For example, water-borne chemicals (“risk cues”) released by predators can cause changes in prey behavior such as feeding rates, thereby altering the impact of the prey species on their own resources. When prey species detect the presence of predators they often alter their behavior by spending more time hiding and generally being inconspicuous, thus reducing the amount of time they spend feeding. The reduced food intake by these prey animals can result in slower growth and reproduction, and ultimately result in population reductions. The reduced feeding rates by the prey then cascade down to release the prey's own food (e.g., plants) from top-down control, causing a trophic cascade based not on changes in prey abundance but by changes in prey behavior. This more subtle mechanism is called “trait-mediated” control. Trait-mediated interactions represent the nonlethal effects of predators, and contrast with the more traditional emphasis on lethal effects. An example of this from the intertidal zones is the effect of risk cues from green crabs that reduce feeding by snails and consequently allow increases in the snails’ food, barnacles and algae. Carnivorous snails exposed to cues from green crabs consumed fewer barnacles, and herbivorous snails exposed to risk cues from crabs consumed far less algae. Both species of snails spent more time in refuges and grew less, while populations of the barnacles and algae increased as a result of reduced top-down control.
Trait-mediated cascades are not restricted to aquatic organisms or environments. Spiders eat grasshoppers, and have direct density-mediated effects on their populations. However, when spiders’ mouths were experimentally glued shut, grasshoppers still showed elevated mortality even though they weren’t being eaten; in the presence of spiders they switched from eating grasses to eating safer, but less nutritious plants, and the reduced energy input led to higher grasshopper mortality. Another way that trait-mediated effects can happen is if the behavioral changes in response to one predator make the prey more vulnerable to another. In fresh water (Freshwater biomes) systems, bass eat darters, and have direct density-mediated effects. To avoid bass, however, the darters tend to hide in rocky shelters, which makes them more susceptible to predation by crayfish that also live in those habitats. Trait-mediated interactions are very common in predator/prey interactions. Some research suggests that the effects of predators on behavior is as important, if not more important, than the direct lethal effects through predation.
Top-down control and inducible defenses
Trait-mediated effects can occur through mechanisms other than behavior. For example, pea aphids, which do not generally have wings, produce winged offspring in response to the presence of ladybird predators or other enemies. This trait modification allows the winged aphids to disperse to new habitats. Water fleas (Daphnia) develop defensive spines when exposed to chemical cues from fish. This makes them more difficult to eat, but developing this structure has energetic costs and reduces lifetime fitness. Snails often develop a thicker shell upon exposure to predacious crabs, which affords the snail greater protection and therefore a greater chance of survival. However, it must expend more energy to carry around a heavier shell. Frog tadpoles in the presence of predators reduce their feeding behavior, but also change their morphology to have a relatively larger tail, which allows them to swim faster and escape predators more effectively. This defense comes at the expense of slower growth. Some of these morphological changes are reversible, but others are not. Crucian carp, when exposed to cues from predatory pike become deeper-bodied, and thus harder for a predator to grab. This body change is reversible.
Plants can also alter their morphology or chemistry in response to herbivores. They may manufacture toxins or noxious chemicals to deter grazing (a number of these chemicals have been shown to have pharmaceutical value). Plants have developed many secondary metabolites involved in defense, which are collectively known as antiherbivory compounds. Defenses in some systems are very complex. For example, Phaseolus plants infested by spider mites emit specific volatile substances which attract predatory mites that eat the spider mites. In addition to chemical defenses, plants may have many external structural defenses that discourage herbivory. A plant's leaves and stem may be covered with sharp spines or hairs, often with barbs, sometimes containing irritants or poisons. After considerable grazing or defoliation, plants can produce a new set of leaves with increased silica and other characteristics that make them tougher and more difficult and less palatable for herbivores to consume.
These altered traits in the presence of predators are referred to as “inducible defenses” or “phenotypic plasticity.” Behavioral, physiological, and morphological plasticity in response to predators can stabilize populations, but the changes can come at a cost. The ultimate effects of top-down processes on the abundance, morphology, behavior, and evolution of populations and communities can be far-reaching.
References
- Borer E.T., Seabloom E.W., Shurin J.B., Anderson K.E., Blanchette C.A., Broitman B., Cooper S.D. & Halpern B.S. 2005. What determines the strength of a trophic cascade? Ecology 86:528-537.
- Miner, B.G., S.E. Sultan, S.G. Morgan, D.K. Padilla and R.A. Relyea 2005. Ecological consequences of phenotypic plasticity. Trends in Ecology and Evolution 20:685-692.
- Pace M.L., Cole J.J., Carpenter S.R. & Kitchell J.F. 1999. Trophic cascades revealed in diverse ecosystems. Trends in Ecology and Evolution 14:483-488.
- Preisser, E.L., D. Bolnick and M.F. Benard 2005. Scared to death? The effects of intimidation and consumption in predator-prey interactions. Ecology 86:501-509.
- Rahel, F. and R. Stein 1988, Complex predator/prey interactions and predator intimidation among crayfish, piscivorous fish, and small benthic fish. Oecologia 75:94-98.
- Schmitz, O. 1998. Direct and indirect effects of predation and predation risk in old-field interaction webs. American Naturalist 151:327-342.
- Schmitz O.J., Krivan V. & Ovadia O. 2004. Trophic cascades: the primacy of trait-mediated indirect interactions. Ecology Letters 7:153-163.