Radon
| Previous Element: Astatine Next Element: Francium |
| |
| Physical Properties | ||
|---|---|---|
| Color | colorless | |
| Phase at Room Temp. | gas | |
| Density (g/cm3) | 0.0097 | |
| Hardness (Mohs) | --- | |
|
Melting Point (K) |
202.2 | |
|
Boiling Point (K) |
211 | |
| Heat of Fusion (kJ/mol) | --- | |
| Heat of Vaporization (kJ/mol) | 18.1 | |
| Heat of Atomization (kJ/mol) | 0 | |
| Thermal Conductivity (J/m sec K) | 0 | |
| Electrical Conductivity (1/mohm cm) | 0 | |
| Source | Synthetic (Ra-226 decay) | |
| Atomic Properties | ||
| Electron Configuration | [Xe]6s24f145d106p6 | |
|
Number of Isotopes |
37 (0 natural) | |
| Electron Affinity (kJ/mol) | --- | |
| First Ionization Energy (kJ/mol) | 1037 | |
| Second Ionization Energy (kJ/mol) | --- | |
| Third Ionization Energy (kJ/mol) | --- | |
| Electronegativity | --- | |
| Polarizability (Å3) | 5.3 | |
| Atomic Weight | 222 | |
| Atomic Volume (cm3/mol) | 50.5 | |
| Ionic Radius2- (pm) | --- | |
| Ionic Radius1- (pm) | --- | |
| Atomic Radius (pm) | --- | |
| Ionic Radius1+ (pm) | --- | |
| Ionic Radius2+ (pm) | --- | |
| Ionic Radius3+ (pm) | --- | |
| Common Oxidation Numbers | --- | |
| Other Oxid. Numbers | --- | |
| Abundance | ||
| In Earth's Crust (mg/kg) | 4.0x10-13 | |
| In Earth's Ocean (mg/L) | 6.0x10-16 | |
| In Human Body (%) | 0% | |
| Regulatory / Health | ||
| CAS Number | 10043-92-2 | |
| OSHA Permissible Exposure Limit | No limits | |
| OSHA PEL Vacated 1989 | No limits | |
|
NIOSH Recommended Exposure Limit |
No limits | |
|
Sources: |
||
Radon is the chemical element of atomic number 86. It is a radioactive, odorless, tasteless noble gas, occurring naturally as the decay product of the element radium; at room temperature it is completely colorless. Radon is one of the densest chemical substances that occurs as a gas under standard temperature and pressure, and is a known health hazard due to its radioactivity. Its most stable isotope, Radon-222, has a half-life of 3.823 days. It has a widespread occurrence due to decay of naturally occurring radium in the Earth's crust; however, there are few molecular compounds known, since it is a noble gas with a complete outer electron shell, and thus does not combine readily with other elements, although radon flouride has been created in the laboratory. The element radon was discovered by Friedrich Ernst Dorn in the year 1900 and originally named niton.
Contents
Physical characteristics
Being a colorless, tasteless and odorless gas, radon is not detectable by human senses. At normal room temperature, radon is a monatomic gas with a density of 9.73 kilograms per cubic meter, or approximately eight the density of ambient air at the Earth's surface; thus radon is one of the heaviest gases at standard temperature and pressure and the heaviest of the discovered noble gases, excluding ununoctium. At standard temperature and pressure, radon is a colorless gas, but when it is cooled below its freezing point of 202 degrees Kelvin, it manifests a brilliant phosphorescence which turns yellow as the temperature is lowered, and eventually becomes orange-red at lower temperatures.[1] Upon condensation, radon also glows because of the intense radiation it produces.
Occurrence
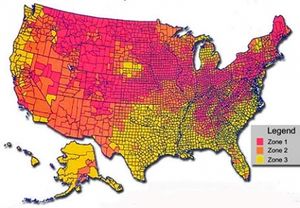
Radon is found widely thoughout the world in the Earth crust and lower atmosphere. Radon, a decay product of uranium, is generally concentrated in ore-bearing rocks, especially granite and Shale. Every square mile of surface soil, to a depth of 15 centimeters contains approximately one gram of radium, which is gradually released in small quantities to the atmosphere; however, due to the short half life, most atmospheric concentrations of radon are relatively near the source of generation, both as to time and place.
Correspondingly there is a wide variation of radon concentration in the atmosphere, depending on location. Above terrestrial locations, it ranges from one to 100 Bq/cubic meter; in certain caverns, mines or poorly ventilated structures above sources, the atmospheric exposure may attain a level magnified by twenty above these air concentrations. Conversely, above oceans or other large surface water bodies the exposure is a minute fraction of this land based exposure. Some examples of areas of very high radon concentration are Carlsbad Caverns, New Mexico; Ramsar, Iran; Yangjiang, China; Ore Mountains of Saxony; locations throughout much of county Mayo and Galway city in Ireland; and mineral springs areas[2]of Boulder, Montana; Misasa, Japan; Bad Kreuznach, Germany.
There are a large number of people worldwide exposed to unacceptably high levels of atmospheric radon. For example, in the United Kingdom, there are over 100,000 homes exposed to dangerous levels of radon gas, with the main concentrations in Cornwall, Derbyshire, Northhamptonshire, Devonshire and Somerset.
Health effects
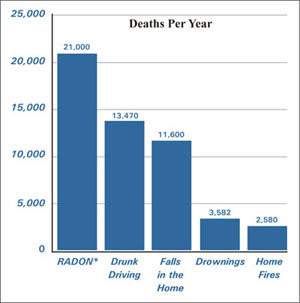
Radon is responsible for the majority of the human exposure to ionizing radiation, frequently being the single largest contributor to an individual's enviornmental radiation dosage. The concentration of radon has a great spatial variability due to its emission from radium rich ores that are locale specific and also due to the short half life, making long distance travel less likely. Radon gas from natural rock sources can accumulate in buildings, especially in confined areas like basements that may lack ventilation. It can also be found in some natural spring waters, including hot springs. Epidemiological data show a clear linkage between breathing high concentrations of radon and incidence of lung cancer. Thus, radon is considered a significant contaminant that affects indoor air quality. Radon is one of the most prominent causes, second to tobacco use, of lung cancer in humans.[3]
Monitoring and control
There is a relatively simple test kit method for measuring radon concentrations. Typically the test cell is set out in the location desired to monitor for 24 hours and then sent to an analytical laboratory. When concentrations are measured at greater than four pico-curies per liter, the space is not recommended for human occupancy according to U.S. EPA standards. Mitigation measures include finding and removing the internal source, if the source is not a naturally occurring crustal radium ore; examples of possible internal building sources are certain granite slabs used for countertops or hearths. If the source is deemed to arise from naturally occurring substrate radium ores, the normal mitigation is to install an active basement ventilation system in the building.
History
Marie and Pierre Pierre Curie noted in 1899 that a radioactive gas emanated from radium; however, discovery of radon is credited to Friedrich Ernst Dorn in the subsequent year. Later in the year 1900 Robert B.Owens and Ernest Rutherford observed vicissitudes when measuring radiation from thorium oxide; these variations were thereafter concluded to be due to the presence of radon gas.
In the year 1904, Sir William Ramsay noticed similarity in the spectra of this new gas with argon, krypton, and xenon, along with the unreactiivity of radon; consequently Ramsay suggested this newly discoved gas to be a member of the noble gas family. In 1910, Ramsay and Robert Whytlaw-Gray isolated radon, measured its density, and concluded that it the heaviest of all gases.