Energy (Energy)
Contents
Energy

(Source: Johannes Jansson, via http://www.norden.org/en/news-and-events/images/topics/energy/sladd/view?gallery=2ccbd5e0c2d4d6c5c2f496643e94400e)
In physics, energy is the capacity for doing work, where work is the action of a force acting over some distance. The term derives from the Greek energeia, or activity, with the first technical definition of energy being provided by Aristotle of Stagira.
Measurement of Energy
Energy is measured in many different ways. The most scientific measurement system is the Système International d'Unités, abbreviated SI. The SI unit of energy is the joule, named for James Prescott Joule, the English physicist who made fundamental contributions to the study of heat and energy. One joule is equal to the work done by a force of one newton acting over a distance of one meter.
Other units of energy are used in specific applications. For example, the energy content of foods is expressed in calories (Cal). The scientific definition of a calorie is the amount of energy needed to raise the temperature of one gram of water from 15° Celsius to 16° Celsius at atmospheric pressure. This is the true calorie, sometimes referred to as a "small calorie". A kilocalorie (kcal) is equal to 1000 calories. In reference to food energy, the term "calorie" is actually 1000 calories or one kilocalorie. One kcal equals 4184 joules. In the British system, energy is often measured in British thermal units (Btu). One Btu is the amount of energy required to raise the temperature of one pound of water one degree Fahrenheit.
The kilowatt-hour (kwh) is usually used to measure the use of electric energy. One kwh is equivalent to one kilowatt of power acting for one hour. One kilowatt hour is equal to 3,600,000 joules.
Physicists and electrochemists often use the electron-volt (eV) to measure energy. The electron volt is the kinetic energy acquired by an electron accelerated from rest through a potential difference of one volt. One eV = 1.602 x 10-19 J.
Forms of Energy
Energy exists in many forms. Electromagnetic energy is the energy associated with electromagnetic radiation of different frequencies (or wavelengths). In order of decreasing frequency (or increasing wavelength, some examples of electromagnetic radiation are gamma rays, x-rays, ultraviolet radiation, visible light, infrared radiation, microwaves, and radio waves. Electricity is energy resulting from the flow of charge particles, such as electrons or ions. Chemical energy is the energy stored in the chemical bonds of molecules. Nuclear energy is the energy stored in the bonds of the sub-atomic particles in the nuclei of atoms. Gravitational energy is energy due to position such as water atop a dam. Sound is a form of energy caused by vibration of atoms and molecules; specifically, the mechanical energy vibrations transmitted as waves through a solid, liquid, or a gas. Mechanical energy is the energy an object has because of its motion or position.
The various forms of energy frequently are classified as either potential or kinetic energy. Potential energy is stored energy or the energy of position. The gravitational energy of water atop a dam is an example of potential energy. Formally, gravitational potential energy is the product of a body's mass (m), the acceleration due to gravity (g), and the the vertical distance of the body from a reference location (h):
PE = m · g · h
Expressing m in kilograms, g in meters per second per second, and h in meters yields units of kilogram · meter2/second2 which is equivalent to joules.
Not all forms of potential energy derive from gravity. Nuclear energy--the energy stored in the nucleus of an atom--is a form of potential energy that can be released when nuclei interact such as in nuclear fusion and nuclear fission. Food, biomass, petroleum, natural gas, and propane are examples of stored chemical potential energy. Elastic potential energy is energy stored as the result of deforming a solid such as stretching a spring. Electrical potential energy is energy associated with electric charges. Albert Einstein's famous equation expresses energy associated with mass as
W = F · d
In the SI system, work is measured in joules when force is expressed in newtons and distance in meters. Note that in a strict physical sense, no work is done unless the object moves. For example, a person pushing against a desk does no work if the desk does not move.
Conversion of Energy
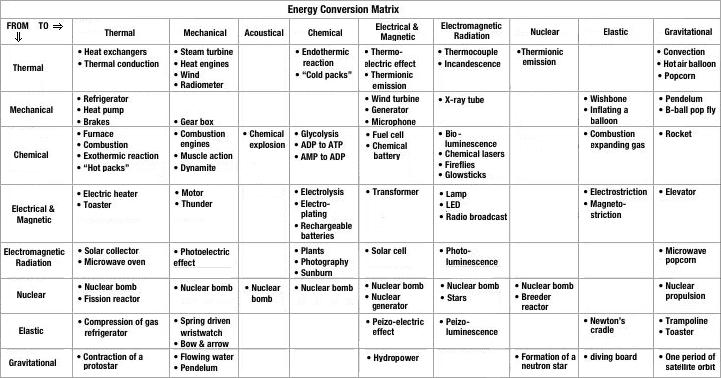
The ability of energy to do useful functions is realized when energy is converted from one form to another via an energy conversion device. Heat for a room is useful and is derived by converting the chemical potential energy in a fossil fuel (natural gas, for example) and oxygen through combustion in a furnace. Similarly, electrical energy is converted to light and heat by a light bulb.
Power
Power is the rate of converting energy or the rate of doing work. Measuring energy or work in joules and time in seconds makes the units of power joules per second or watts (W). A 60 watt light bulb converts electric energy at a rate of 60 joules per second. The watt is named for James Watt, renowned for his improvements on the steam engine.
Horsepower is another measure of power that frequently is used to measure the rate at which an engine or motor does work. One horsepower equals 746 watts. An electric motor on a small household appliance produces about 1/6 horsepower, a healthy human can sustain about 0.1 horsepower, a car can generate several hundred horsepower, while a steam turbine in an electric power plant can produce more than 1.5 million horsepower.
Electricity
In a direct current circuit using batteries, for example, electric power is the product of potential difference measured in volts and current measured in amperes. In an alternating current circuit such as in a household electric power is the product of the effective values of the voltage and current.
Conservation of Energy and Efficiency
One of the fundamental principles of science is that energy can be converted from one form to another, but the energy before the conversion equals the energy after the conversion. This is known as the conservation of energy, or the first law of thermodynamics. We can use the different forms of energy discussed above to define an object's total energy, E, as the sum of the object's kinetic (KE), potential (PE), and thermal energy (TE):
E = KE + PE + TE
A light bulb uses electricity and produces both heat and light. If 100 joules of electricity are converted by the bulb, perhaps only 10 joules are converted to light, with the remaining 90 joules converted to heat. But consistent with the conservation law, the total energy out in the form of heat and light equals the energy input in the form of electricity. The form or quality of energy changes, but the quantity remains the same.
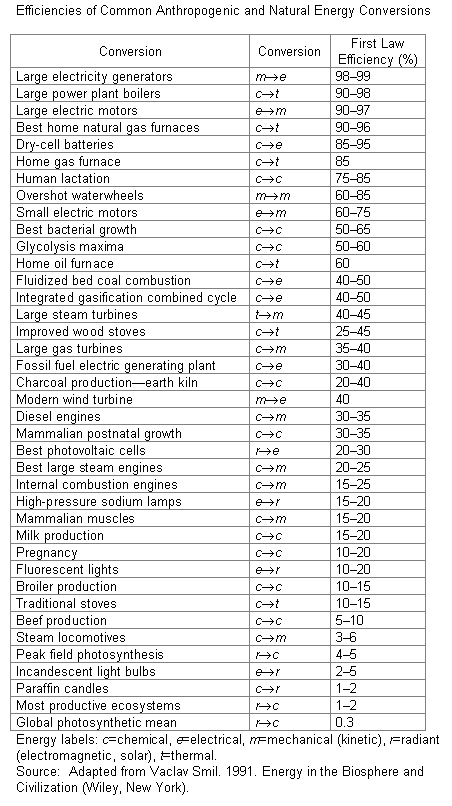
Physicists and engineers frequently are interested in the fraction or percentage of energy converted in any process to useful forms. This fraction is called the efficiency and is defined as:
efficiency = useful energy out/total energy converted
This fraction, which also is known as the first law efficiency, is always less than one because the energy out is always less than the energy input. In the incandescent light bulb example, the first law efficiency is:
5 joules of light/100 joules electricity = 0.05 or 5%
Note that efficiency is a dimensionless number, i.e., it has no units because the numerator and denominator are measured in energy units such as joules. Any unit of energy can be used as long as the energy inputs and energy output are measured in the same units.
The first law efficiency of some important energy conversion devices are shown in the adjacent table.
Heat and Work
Temperature and Heat
In a qualitative sense, the temperature of an object determines the sensation of warmth or coldness felt from contact with it. A more rigourous definition is that temperature is the average kinetic energy of the atoms and molecules in a substance. When a high temperature object is placed in contact with a low temperature object, then energy will flow from the high temperature object to the lower temperature object, and they will approach an equilibrium temperature. Note that temperature is not directly proportional to internal energy because temperature measures only the kinetic energy part of the internal energy, so two objects with the same temperature do not in general have the same internal energy]. The internal energy may be increased by transferring energy to the object from a higher temperature (hotter) object, a process called heating.
There are three main temperature scales used in the world - Celsius, Fahrenheit, and Kelvin. These are compared in the following table.
| Temperature Scale comparisons | |||
|---|---|---|---|
| °C | K | °F | |
| Water boils | 100 | 373 | 212 |
| Water freezes | 0 | 273 | 32 |
| Absolute zero | -273 | 0 | -459 |
The relationship between temperature and heat is described by the concept of specific heat, which is the amount of heat required to change a unit mass of a substance by one degree in temperature. An equivalent statement is that specific heat is the amount of heat a material can hold when its temperature is raised one degree C.
Q = m · c · ΔT
where m = mass in kilograms (kg), c = specific heat heat in joules per kilogram-degree centigrade (J/kg/°C) and T = degrees Centrigrade.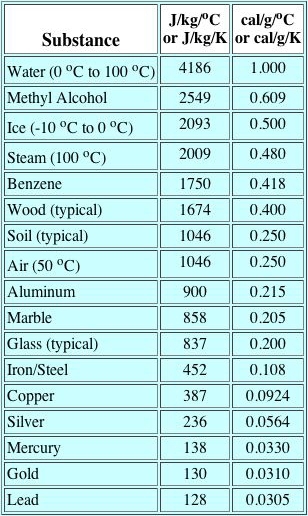
This is a very important feature in our lives. Some other important definitions related to energy, temperature, and heat are:
- Heat Capacity - of a substance is the ratio of the amount of heat energy absorbed by that substance to its corresponding temperature rise.
- Sensible Heat - is heat that can be measured by a thermometer, and thus sensed by humans. Several different scales of measurement exist for measuring sensible heat. The most common are: Celsius scale, Fahrenheit scale, and the Kelvin scale.
- Latent Heat - is the energy required to change a substance to a higher state of matter. This same energy is released from the substance when the change of state is reversed. The diagram below describes the various exchanges of heat involved with 1 gram of water.