Antarctic ozone hole
Contents
The Ozone Hole
The term ozone hole or Antarctic ozone hole refers to the seasonal depletion of stratospheric ozone in a large area over Antarctica. The chemistry behind the formation of the ozone hole was described by F. Sherwood Rowland, Mario Molina and Paul Crutzen, who in 1995 were awarded the Nobel Prize in Chemistry “for their work in atmospheric chemistry, particularly concerning the formation and decomposition of ozone.”
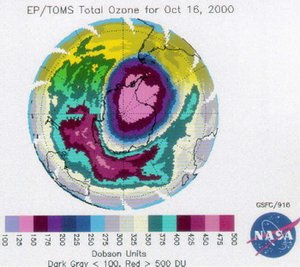
From satellite images and Dobson Spectrometer measurements, it became clear in the period around 1980-1985 that a substantial part of stratospheric ozone in Antarctica was disappearing during the local springtime (Figure 1). (A Dobson Spectrometer measures solar light intensity at a wavelength at which ozone absorbs light, and at one where ozone does not absorb light. The total ozone column between the apparatus and the sun is derived from the difference of the measurements.) It was determined that CFCs (chlorofluorocarbon compounds) used in refrigerators, air conditioners, and spray cans, were the main cause. Less stratospheric ozone results in more shortwave ultraviolet (UV) radiation reaching the Earth's surface. This can affect human health by causing skin cancer and can also potentially affect marine ecosystems (Marine ecosystem services) when algae populations, the basis of the oceanic food chain, are damaged. The problem, it was feared, would not be restricted to Antarctica and would become an environmental threat on a global scale.
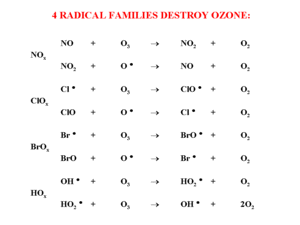
Four different families of radicals play an important role in ozone depletion (Figure 2). In general, electrons in compounds are grouped in pairs. Radicals have a lone electron and this makes them very reactive. The reaction schemes make it clear that the radical species, which are responsible for the ozone destruction, reform: one radical can destroy many ozone molecules. The radical is not used, and as long as they are not destroyed by other reactions, they act as a catalyst, not as a reactant.
Sources of Radicals
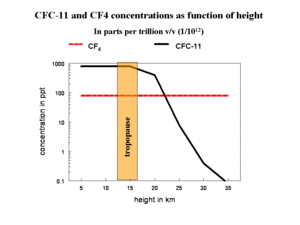
Atmospheric research quickly revealed that the chloro-, bromo- and part of the nitric oxide (NO) radicals entered the stratosphere because the original compounds from which they are formed are not destroyed by OH-radicals in the troposphere, as happens with most organic and inorganic gaseous pollutants. The concentration of CFC-11 (formula CCl3F) is constant as a function of height in the troposphere, as CFC-11 is not destroyed by OH-radicals (Figure 3). Eventually, CFC-11 enters the stratosphere and is destroyed by the very short wavelength UV, which is filtered out by stratospheric ozone. CF4 is stable even in the lower layers of the stratosphere. NO in the stratosphere is largely the result of destruction of N2O by shortwave UV.
The reactivity of the radicals decreases in the order of:
BrOx > ClOx > NO > OH
The BrOx concentrations are quite low and the largest contribution to ozone destruction comes from chloro-radicals. The majority of these chloro-radicals (probably more than 90%) is produced by human activity. CCl3H and HCl also contribute to ozone depletion and could partly be coming from natural sources.
Extensive laboratory research and measurements regarding the Antarctic ozone hole indicated that such a rapid and extensive ozone destruction could not be explained with the knowledge of the atmospheric gas-phase chemistry of that time. It finally became clear that particles in the stratosphere play a key role in the chemistry of the polar atmosphere (Figure 4). From field measurements, it appeared that rapid chlorine-catalyzed ozone destruction was taking place in the Antarctic stratosphere during the polar winter and early spring as a consequence of high ClO concentrations. These high ClO concentrations are formed by reactions on the surface of water and nitric acid aerosols existing as ice-clouds ("Polar Stratospheric Clouds").
The particles collect at very low temperatures during the winter radical species on their surface, and release these radicals in early Spring as the temperature rises. The particle surface acts as a catalyst, and this, along with the fact that concentrations of radicals are elevated in the direct vicinity of the particles, leads to rapid ozone depletion.
In Winter and early Spring a rotating air mass, the so-called Antarctic Vortex, is formed. This air mass is uncoupled with the global weather system and very little air is exchanged. Because of this, the destroyed ozone cannot be replenished, resulting in the Antarctic ozone hole.
Effects of Stratospheric Ozone Loss
Ozone absorbs solar energy that is dangerous to living organisms. Solar radiation that reaches the outer layer of the atmosphere (Atmosphere layers) can be grouped into three categories according to its strength or intensity (Figure 5). Solar radiation with the shortest wavelength, ultraviolet radiation A (UV-A), is the most dangerous because it has the highest energy. Radiation in this category is absorbed strongly by the atmosphere so that very little reaches the surface, regardless of the thickness of the ozone layer. Another category of radiation with longer wavelengths is absorbed and used by plants in photosynthesis, and is known as photosynthetically active radiation. A significant fraction of photosynthetically active radiation reaches Earth's surface, a condition that enables life to exist.
Solar radiation with wavelengths between these two categories is ultraviolet radiation B (UV-B). The amount of UV-B that reaches Earth's surface depends in part on the concentration of ozone in the stratosphere. As the concentration of ozone has declined, the amount of biologically significant UV-B radiation that reaches Earth’ surface has increased. Since 1980, the amount of UV-B that reaches the surface has increased 4-7 percent increase in the Southern Hemisphere, increased 6 percent at the middle latitudes of the Southern Hemisphere, and increased 130 and 22 percent in the Antarctic and Arctic spring-time respectively.
These increases merit attention because they pose a threat to living organisms. UV-B's relatively short wavelength allows it to penetrate deep into living tissue. There, it can cause considerable damage by breaking apart molecules. Exposure to high levels of UV-B can cause death. Lesser amounts can cause a variety of damages. For example, increased levels of UV-B radiation impair the function of enzymes that are part of the photosynthetic process in several tree species. Breaks and rearrangements of DNA molecules are called mutations. Mutations are especially dangerous for living organisms because DNA contains the information that is needed to make the components of the body, to turn on and off cell growth, and to reproduce.
Impact on Marine Ecosystems along Antarctica
The vulnerability of phytoplankton in general and the timing and location of the greatest increases in ground-level UV-B radiation make marine ecosystems along Antarctica especially vulnerable. Blooms occur at the edge of the Antarctic ice pack, where melting ice forms a shallow layer of relatively fresh water. Because fresh water is less dense than salt water, it floats on top of the salt water. This area is known as the marginal ice zone. The marginal ice zone is ideal for phytoplankton because there is plenty of light and nutrients. But this habitat makes phytoplankton vulnerable to UV-B. Because the marginal ice zone is shallow, the phytoplankton cannot escape from the UV-B radiation by moving to deeper water.
The increase in UV-B may decrease net primary production, alter food webs, and slow biogeochemical cycles. Laboratory experiments indicate that the increase in UV-B radiation could reduce net primary production by about 25 percent if phytoplankton have no defense mechanisms. But most phytoplankton do have some mechanism to cope with higher levels of UV-B. Studies of phytoplankton in the ocean indicate that the increase in UV-B may reduce net primary production by up to 10 percent.
The increase in UV-B also may change the composition of phytoplankton community. Such changes are important because not all species of phytoplankton are edible by zooplankton. That is, zooplankton can eat only certain types of phytoplankton. If the composition of phytoplankton changes in favor of less-edible species, the amount of energy that flows through the food chain could be reduced by an amount that is greater than the reduction in net primary production. This could change the entire food chain.
Changes in the Antarctic marine ecosystem could ripple across the entire planet. Ecologists estimate that net primary production in seas around Antarctica account for 10-20 percent of the total generated by the world's oceans. This production supports marine and terrestrial ecosystems well beyond the southern pole. Many birds and marine mammals migrate to Antarctica and rear their young to take advantage of the abundant food supplies in the spring. Should net primary production diminish, the energy return on investment for these migrations would be reduced. That would leave less surplus energy to produce and raise offspring.
The increase in UV-B also could alter biogeochemical cycles. Bacteria are an important component of the nitrogen and sulfur cycles. Bacteria generally are more vulnerable to UV-B radiation; therefore reductions in stratospheric ozone could reduce microbial activity, such as decomposition. This would increase the time that nutrients remain tied up in dead organic matter. This, too, would slow several important biogeochemical cycles.
The Impact on Terrestrial Organisms (Including People)
Terrestrial ecosystems on Antarctica are very sparse, so the reduction in stratospheric ozone should have a relatively small effect on plants and animals. On the other hand, spring-time break-up of the polar vortex and the subsequent spread of ozone-poor air over the Southern Hemisphere could have a significant effect on terrestrial ecosystems, including people.
Similar to its effect on marine ecosystems (Marine ecosystem services), a reduction in stratospheric ozone could reduce net primary production by terrestrial ecosystems. Leaves of conifer trees generally are less vulnerable to UV-B than the leaves of deciduous trees and herbaceous plants. More UV-B radiation slows leaf elongation rates, reduces leaf area, and reduces above-ground biomass. Nonetheless, these reductions are expected to be small relative to aquatic ecosystems because protective mechanisms by plants are more effective.
Photosynthesizers are not the only vulnerable organisms. Many animals lay eggs and pass through juvenile stages that are vulnerable to UV-B radiation. Some herpetologists (scientists who study amphibians and reptiles) think that some of the world-wide reduction in frog and toad populations may be caused in part by a reduction in stratospheric ozone. A study in Oregon indicated that elevated levels of UV-B reduced the fraction of eggs that hatched. Species that had the lowest success had the least ability to repair mutations.
Nor does this effect have to be so direct. Effects of elevated levels of UV-B radiation may be exacerbated by climate change (Causes of climate change). Embryos of some amphibian species are vulnerable to fatal fungal infections following exposure to UV-B radiation. Exposure is determined both by ozone concentrations and water depth (water absorbs UV-B radiation, so that eggs laid in deep waters are more protected). Water levels in ponds of the Pacific Northwest are relatively low during El Niño events (the change in atmospheric circulation steers storms away from the Pacific Northwest towards southern California) and this increases the mortality rates for amphibian embryos. If climate change (Causes of climate change) increases the frequency of El Niño events and/or warms and dries the Pacific Northwest, these changes would enhance the exposure to UV-B radiation that is associated with a reduction in stratospheric ozone.
Finally, UV-B radiation can have direct health effects on terrestrial animals, including people. Research indicates that increased levels of UV-B damage internal organs, suppress the immune system, and increase the formation of cataracts and lead to blindness. The most visible concern for human health focuses on skin cancer. The U.S. Environmental Protection Agency estimates that every 1 percent reduction in the ozone concentrations increases basal and squamous-cell skin cancers (the least dangerous kinds) by 2-3 percent and increases malignant melanoma skin cancers (the most dangerous kind) by 1-2 percent. Trends in the rate of skin cancer seem to justify this concern. Melanoma was the fastest growing cancer in the U.S. population of Caucasians, prior to implementation of the Montreal Protocol.
But is this increase is caused by the reduction in stratospheric ozone? Changing fashion also could be responsible. During the last thirty years, exposure to UV-B radiation has increased as people pursue the perfect tan. A tan is viewed as a status symbol that indicates a person can travel to warmer climates during the winter and/or has sufficient leisure time to lie around in the sun. This attitude contrasts to earlier times, when pale skin was the ideal. Pale skin indicated that you didn’t have to work out in the sun (i.e., worked on a farm)—you worked in an office or could spend all your time lounging around the house. Because of this change in fashion, scientists can’t say if the rise in skin cancer is due to ozone depletion or people having more fun in the sun. Undoubtedly, both are important.
Montreal Protocol
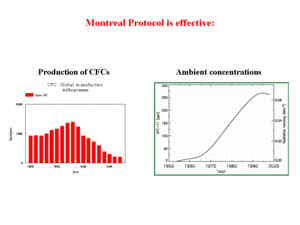
The direct threat to human health has prompted a series of conferences focused on reducing the emissions of the CFCs responsible for ozone depletion. These talks resulted in the Montreal Protocol on substances that deplete the ozone layer, which phases out the manufacture, sale, and use of these compounds. The negotiations were strictly based on a scientific analysis of the situation and this basis proved to be very effective. In fact, the use of CFCs was reduced effectively as a result of the Montreal Protocol (Figure 6). In view of the long time needed for these compounds to traverse the tropopause, the time lag between CFC emissions reduction and actual stabilization and reduction of ambient concentrations of CFCs is quite large. Nonetheless, recent measurements indicate that the concentrations have indeed stabilized and are even declining, although at a slow rate.The Montreal Protocol, and the efficiency of the measures taken, is a good example of how environmental policy, based on a sound scientific basis, can be very effective. The approach taken under the Protocol is similar to those under the UN-EMEP (United Nations European Monitoring and Evaluation Programme) protocols in Europe. To begin with, agreement was reached on the methods for describing the possible threat. Next, agreement was reached on possible abatement methods and replacements for the use of specific CFCs. Only after agreement was reached on all methodologies to be applied, was the evaluation actually carried out. The fact that developed and politically strong countries such as Australia would be affected by ozone depletion may have also been key to the success of the Montreal Protocol.
References
- Antarctic climate and environment history in the pre-instrumental period. (2021) Editors: Luca Bargelloni, John Turner et al. Victoire Press. Cambridge, UK. Published by the Scientific Committee on Antarctic ResearchScott Polar Research Institute, Lensfield Road,Cambridge, UK. ISBN 978-0-948277-22-1
- Gordon W. Gribble. 1998. Naturally Occurring Organohalogen Compounds. Acc. Chem. Res. vol.31, issue 3, pp 141–152
- Stratospheric Ozone Depletion by Chlorofluorocarbons (Nobel Lecture) by F. Sherwood Rowland
Citation
Sjaak Slanina, Cutler Cleveland & Robert Kaufmann (2009). Antarctic ozone hole. eds.Howard Hanson & C. Michael Hogan. Encyclopedia of Earth. National Council for Science and Environment. Washington DC. Retrieved from http://editors.eol.org/eoearth/wiki/Antarctic_ozone_hole