Mercury recycling in the United States in 2000
Contents
- 1 Introduction The purpose of this report is to summarize recycling (Mercury recycling in the United States in 2000) of elemental mercury in the United States in 2000. For this report, the term “mercury” implies elemental mercury, which may variously be classified as a commodity, chloralkali waste or sludge, industrial waste, or toxic or hazardous waste in domestic or international commerce.
- 2 Global geologic occurrence
- 3 Primary production and processes
- 4 Sources of secondary mercury
- 4.1 Old scrap
- 4.1.1 Automobile convenience switches
- 4.1.2 Dental amalgam
- 4.1.3 Fluorescent Lamps
- 4.1.4 Lab/Medical
- 4.1.5 Thermostats
- 4.1.6 Chlorine-Caustic Soda Industry
- 4.1.7 Other Uses
- 4.1.8 Imports and exports with unspecified mercury content
- 4.1.9 U.S. Government Mercury Stockpile
- 4.1.10 Discontinued and dissipative uses
- 4.2 New scrap generated
- 4.1 Old scrap
- 5 Disposition of mercury scrap in landfills
- 6 Old scrap recycling efficiency
- 7 Infrastructure and processing
- 8 Outlook
- 9 Further reading
- 10 Attached Files
Introduction The purpose of this report is to summarize recycling (Mercury recycling in the United States in 2000) of elemental mercury in the United States in 2000. For this report, the term “mercury” implies elemental mercury, which may variously be classified as a commodity, chloralkali waste or sludge, industrial waste, or toxic or hazardous waste in domestic or international commerce.
According to the U.S. Geological Survey (USGS) Minerals Yearbook, data for secondary production, or recycling, of mercury date to the 1940s. Large-scale recycling of mercury took place in the 1950s when 10,900 t of mercury was taken to Oak Ridge National Laboratory in Alabama for production of the hydrogen bomb and most of the mercury was returned to the General Services Administration. A study by Jasinksi indicated that widespread mercury recycling did not begin until about 1989-90.
Commonly used mercury products that may be recycled include automobile convenience switches, dental amalgam, laboratory/medical devices, which include thermometers, fluorescent lamps, and thermostats. For example, approximately 20 to 23 percent of mercury-containing fluorescent lamps are recycled. The overall use of mercury, however, is declining. Therefore, each year fewer and fewer mercury-containing products, or secondary mercury sources, are available for mercury reclamation and recycling.
A number of mercury-containing devices are routinely used in building construction and for heating, ventilation, and air-conditioning (HVAC) systems. Although mercury is widely used in chemical compounds and solutions, mercury in these materials is not recycled. Although the use of mercury in batteries and paints has generally been discontinued in the United States, some mercury-containing batteries can be imported.
Ever-increasing human health and environmental concerns, liability issues associated with hazardous waste removal and treatment, and efforts to prevent mercury-containing waste from being sent to landfills show the importance of the mercury recycling industry. Businesses may face fines and prosecution if mercury-containing products are improperly disposed of after use. Local and State environmental regulations require adherence to the Resource Conservation and Recovery Act (RCRA) and subsequent amendments and the Comprehensive Environmental Response, Compensation, and Liability Act, which is also known as Superfund, to regulate the generation, treatment, and disposal of mercury as hazardous waste.
Mercury has had widespread worldwide use in the chlorine-caustic soda industry since the 1950s. Nonmercury technologies, such as the diaphragm and membrane cells, however, now provide alternative methods for producing chlorine-caustic soda. Mercury is also used in a variety of products that include batteries in some children’s athletic shoes and toys that light up or make noise, in computer electronics, and as a fungicidal component of rubber flooring used in gymnasiums. The Connecticut Attorney General asked cereal manufacturers to remove boxes of cereal that contained light-up toys with mercury batteries from store shelves. The materials flow of mercury in international and domestic economies is described in Sznopek and Goonan, and the present and future world supplies and demands for mercury are discussed in Weiler.
Estimating the amount of mercury that is recycled in the United States is inherently difficult. For example, chlorine-caustic soda production routinely produces sodium-laden mercury amalgam, which is recycled in-plant as home scrap. In 1990, approximately two-thirds of recycling, in strict terms of mercury recovered and reused, involved in-plant recycling of mercury, as home scrap, in the chlorine-caustic soda industry. Mercury used for other applications may be recycled, and many mercury-containing products, such as automobile switches and fluorescent lamps, may become part of a landfill. Breakage of fluorescent lamps en route to a recycling station or landfill can release mercury into the environment. The amount of mercury that is recycled is low, but the share of apparent supply that is scrap (excluding imports and exports for which estimates could not be made) is relatively high.
Improved analytical techniques and research have made it possible for industries to recover and conserve mercury that otherwise could escape into the environment. The study of mercury releases is a global research priority. Because of its role as an important mineral commodity and its effect on the environment, the materials flow of mercury was extensively covered in a U.S. Geological Survey (USGS) publication on materials in the economy.
In the 1950s, the effect of mercury on human health was tragically dramatized when 50 people died and many more were poisoned from eating mercury-contaminated seafood from Minamata Bay, Japan. In the early 1970s, mercury-treated seed grain was consumed rather than planted, which resulted in more than 400 deaths in Iraq. Concern about the level of mercury in fetal blood owing to consumption of contaminated fish is rising.
The voluntary closure of domestic chlorine-caustic soda plants that use mercury and the subsequent disposition and management of the approximate 3,000 metric tons (t) of mercury contained in these plants is another serious concern. There are 80 chlorine-caustic soda plants in Europe, and 48 of these are mercury-based. The date for closure of these mercury cell plants is voluntary and is expected to be no later than 2020. It is estimated that there is 11,500 t of mercury in the cells. The effect of the closure of European chlorine-caustic soda plants and the disposition of the mercury used in those plants was presented in a report by the European Commission and in a recent report on the global chlorine-caustic soda industry by Winalski and others.
Mercury and mercury-containing products, such as computers, dental amalgam, and fluorescent lamps, are recycled by AERC Recycling in Pennsylvania; Bethlehem Apparatus Co. in Pennsylvania; D.F. Goldsmith Chemical and Metal Corp. in Illinois; Mercury Waste Solutions, Inc. in Minnesota; Onyx Environmental Services in Wisconsin; and USA Lights in Maryland among others. A list of more than 50 individuals and companies involved mainly in the collection stages of mercury recycling is listed by Mercury Recyclers, and a list of mercury collection organizations is provided by Hospitals for a Healthy Environment and the Ohio Office of Pollution Prevention. In 1998, 12 companies actually retorted the collected mercury-containing material. By 2002, that number had dropped to only five. The mercury recycling industry has been deeply affected by the increase in shipments of mercury-containing waste to Canada where the materials may be landfilled.
|
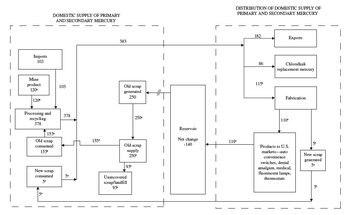
Table 1: Salient statistics for U.S. mercury scrap in 2000 Metric tons unless otherwise specified; $150 per flask or $4,350 per metric ton in 2000. t, metric tons; NA, not available Old scrap: Generated1 250 t Consumed2 155 t Consumption value3 $670,000 Recycling efficiency4 62 percent Supply5 250 t Unrecovered6 95 t New scrap consumed7 5 t New-to-old scrap ration8 3:97 Recycling rate9 80 percent U.S. net exports of scrap10 NA Value of U.S. net exports of scrap3 NA Mercury content of products theoretically becoming obsolete in the United States in 2000. Mercury content of products that were recycled in 2000. Value of mercury scrap based on primary mercury price. (Old scrap consumed plus old scrap exported) divided by (old scrap generated plus old scrap imported plus any old scrap stock decrease or minus any old scrap increase. Some items were not available. Old scrap generated plus old scrap imported (not available) plus old scrap stock decrease (not available). Old scrap supply minus old scrap consumed minus old scrap exported (not available) minus old scrap stock increase (not available). Including new industrial scrap but excluding home scrap. Ratio of quantities consumed, in percent. Fraction of the apparent metal supply that is scrap, on annual basis. Trade excluded in apparent supply calculation because no reliable estimates are available on mercury content of imports or exports. Trade in scrap is assumed to be principally in old scrap. Net exports of old scrap minus imports of old scrap (not available). Much of the data in Figure 1 is estimated, and the values and volumes of each category of mercury-containing material described herein have the potential for dramatic change from year to year. The average price of mercury (Mercury recycling in the United States in 2000) was approximately $150 per flask from 2000 until 2003 and then rose sharply to $650 per flask in fall 2004 and approximately $850 per flask in spring 2005. Figure 1 shows estimated mercury materials flow in 2000, unless otherwise indicated, with estimates given for old and new scrap consumption and old scrap generated. Table 1 lists salient statistics based on data in the flow chart. The authors would like to thank Thomas Downing, facility manager, AERC Recycling, Ashland, Va.; Bruce Lawrence, President, Bethlehem Apparatus, Hellertown, Pa.; David Goldsmith, President, D.F. Goldsmith Chemical and Metal Corporation, Evanston, Ill.; Brad Buscher, chairman, and staff, Mercury Waste Solutions, Mankato, Minn.; Michael Merry, logistics superintendent, Minera Barrick Misquichilca, SA, Lima, Peru; and Richard Fortuna, President, Strategic Environmental Analysis, Potomac, Md., for arranging site visits and providing review comments and documents for this report. Global geologic occurrenceMercury is a scarce metal that is liquid at room temperature and is obtained primarily from the red mineral cinnabar, which is a mercury sulfide (HgS). It averages 0.05 part per million in the Earth’s crust. A complete list of the chemical and physical properties, isotopes, and thermodynamic properties of mercury is given by DeVito. Elemental mercury, which is also known as azogue or quicksilver, was known to Aristotle in the 4th century B.C. “Hydrargyrum” is the ancient Greek word from which the chemical symbol “Hg” is derived, and this word is still used, though rarely, today. Sources of cinnabar and accounts of shipments of cinnabar from the Almaden (Arabic for mine) region in Spain to Rome are given in Goldwater and Putnam. Agricola described several methods for retorting the ore and recognized that exposure to mercury vapors during retorting caused the teeth to become loose. In Peru, the Inca recognized the health hazards of mercury, or “llimpi,” and that exposure to mercury vapors caused by firesetting, which was an ancient mining technique, in the mercury mines at Huancavelica caused the ancient Andean miners to tremble and shake. Mercury from Almaden was used for expansion of Spain’s silver-processing capabilities in Bolivia and Mexico during the 1500s. Exploitation of the cinnabar deposits at Huancavelica in the mid-1500s by Spanish explorers provided a regional source for this metal, which was vital to Spanish colonial silver processing in the Americas. The more-common ore of mercury, cinnabar, is dark red and soft, and may be associated with low sulfidation epithermal mineral deposits worldwide; this is a specific type of hydrothermal mineral occurrence that forms at depths of less than 1 kilometer and at temperatures of less than 300° C. Mercury, along with arsenic and antimony, is used as a geochemical exploration guide for precious and base metals at depth. Mercury ores may be found disseminated in fine-grained or brecciated sedimentary and volcanic rocks near volcanic centers, fossil hot-springs, and intrusive rocks and may be any age from Silurian to Tertiary. The United States has numerous mercury occurrences. A bibliography of mercury occurrences in many countries of the world, such as Bolivia, Canada, China, Russia, and Venezuela, is given in Ebner. Mercury is retorted from cinnabar, may occur as a native metal, or is produced as a byproduct of copper (tennantite-tetrahedrite), gold (amalgam), lead-zinc (sphalerite), and silver (kongsbergite) smelting. Coal-fired power plants in the United States are another source of mercury releases to the atmosphere. Mercury may also be found in the copper concentrates and in the dust, fly ash, and other wastes associated with copper smelting. In 1998, an estimated 650 kilograms (kg) of mercury was released into the atmosphere as the result of precious-metal smelting at one mine in Nevada. Several flasks of byproduct mercury that were produced during gold smelting were spilled during transport in a small town in Peru in 2000. Primary production and processesMercury has not been mined domestically as a primary mineral commodity since the closure of the McDermitt Mine, Nevada, in 1992. Data on byproduct mercury, which is produced mainly from gold mining and processing, are estimated in this report. In 1992, nine gold mines in the United States were producing and reporting their byproduct mercury production. The gold mining industry is the primary source of new elemental mercury as a byproduct of gold processing. Byproduct mercury from Peru’s Pierina gold mine is recovered, carefully packed, and shipped to the United States for recycling. All handlers of this mercury, even customs agents, receive training in the safe handling of mercury. Van Zyl and Eurick projected that a minimum of 18 t of byproduct mercury would be produced from gold mines in Nevada in 2000. Sales of mercury retort systems permit the inference that byproduct mercury is routinely recovered at a minimum of six gold mines in Nevada. Calomel (Hg2Cl2), which is a mineral and a mercury-bearing byproduct released during gold processing, may be captured by polltion-control devices at smelters and retorted to recover mercury. In the future, mercury from coal-fired power plants may be recovered as a byproduct. Researchers at the Illinois Institute of Technology have shown that gold-carbon filters remove 98 percent of the mercury from powerplant test emissions and that the filter can be recycled and the mercury reclaimed. Canada has not produced mercury as a principal commodity since 1975. Finland produces approximately 50 metric tons per year (t/yr) of byproduct mercury from zinc smelting, and Sweden considers the 20 t/yr of mercury produced from copper smelting to be toxic waste. The Swiss company Batrec, which was founded in 1989, is Europe’s leading recycler of mercury-containing materials. It processes more than 3,000 t/yr of batteries, sludges, and other materials. The Mercury Recycling Group, which is the largest recycler of mercury in the United Kingdom, has tripled its recycling capacity in response to European environmental legislation. In Barcelona, Spain, hardware stores serve as recycling pickup stations by collecting batteries and fluorescent lamps in cardboard containers. Spanish consumers may call a telephone number (900 30 05 06) for information on recycling and lamp collection. In Australia, several mercury cell chlorine-caustic soda plants were closed at the end of 2000 because plants that use nonmercury technology were opened, thereby releasing that mercury onto the global market for reclamation and recycling. In 2002, for example, the United States imported 107 t of mercury from Australia as a result of the closure of these plants. In 2000, the United States imported 103 t of mercury, mainly from Australia (25 t) and Germany (25 t) and exported 182 t of mercury mainly to India (65 t) and the Netherlands (51 t). Additional mercury was sent out of the United States as chloralkali waste from a decommissioned chloralkali plant in Maine to India. The United States has 4,436 t of mercury stockpiled by the Defense Logistics Agency. On a global scale, cinnabar is mined by open pit or underground mining, and most ore is recovered from depths of less than 350 meters. Industry projections indicated that 25,000 flasks (900 t) would be produced from the mines at Almaden, Spain, by the end of July 2004. Gold mining companies in Nevada, which represented most U.S. production in the early 1990s, voluntarily provided byproduct mercury production data to the U.S. Geological Survey for 1990 (114 t), 1991 (58 t), and 1992 (64 t); the average byproduct mercury production for that 3-year period was 79 t. Data for other years are not available. No information is available on byproduct mercury produced from any other potential domestic sources, such as precious- or base-metal mining and processing in 2000. Van Zyl and Eurick estimated Nevada byproduct mercury production to be 13 t for 1999 and projected that 18 t of byproduct mercury would be produced in 2000. Their estimates are less than the average amount of byproduct mercury reported to the USGS between 1990 and 1992. In 2000, reported world mercury mine production was concentrated in Spain (500 t), Kyrgyzstan (257 t), Algeria (216 t), and China (200 t). Producing countries may be reluctant to report their production data of primary or byproduct mercury because of increasing concern about environmental effects, global human health concerns, and liability issues. For example, in 1972, primary mercury was produced in 22 countries; that number had fallen to 11 by 1987. Since that time, primary mercury production has generally decreased owing to the availability of byproduct mercury, environmental concerns, low demand, recycled mercury, and, until recently, low prices. In general, mercury ores may contain from 0.1 to more than 2 percent mercury; most economic ores contain more than 1 percent mercury. The ore is crushed, screened, and then heated in a retort or furnace with limited ore beneficiation. Approximately 95 percent of the mercury contained in the ore can be recovered at commercial grade (99.9 percent purity) by this method. Other specialized production methods include leaching, dissolution, and electro-oxidation. Sources of secondary mercuryOld scrapDiscarded mercury-containing products, such as automobile convenience switches, dental amalgam, lab/medical devices and thermometers, fluorescent lamps, and thermostats, are the main sources of old mercury scrap. Miscellaneous electronics, batteries, computers, chlorine-caustic soda production debris, demolition debris, fungicidal gym flooring, light-up tennis shoes, and any mercury-contaminated materials are other sources of old scrap. Mercury Waste Solutions provides a detailed list of more than 50 acceptable materials for recycling. Some of these products are recycled; on a national scale, however, mercury-containing products more commonly become part of a landfill. Automobile convenience switchesIn 1995, 14 million mercury switches, or nearly 12 t of mercury, were used for active ride control systems, antilock braking systems, and hood and trunk convenience lighting. In 2000, U.S. automakers used an estimated 4 million 1-gram (g) mercury switches. Mercury was phased out of automobile switches manufactured outside of the United States in 1996. In 2003, the European Union inaugurated a directive that prohibits cadmium, hexavalent chromium, lead, and mercury to be used in the manufacture of autos . Therefore, 1996-and-later foreign-manufactured vehicles that enter the domestic scrap recycling stream are not sources of old scrap mercury. An estimated 150 to 200 t of mercury is still contained in the entire fleet of 210 million to 250 million vehicles in the United States and up to 10 t/yr of mercury may be released from shredded vehicles. Names of domestic vehicles manufactured with mercury switches are available from the U.S. Environmental Protection Agency and the Clean Car Campaign. Information and step-by-step instructions on how to find, remove, and replace mercury switches in domestic vehicles is provided by the U.S. Environmental Protection Agency. The Alliance of Automobile Manufacturers indicated that an automobile recycling facility that processes 500 cars per year can theoretically store 10 year’s worth of convenience switches in a 1-gallon pail. The industry is very concerned regarding who is ultimately responsible for the removal of the mercury-containing switches before automobiles are shredded—the manufacturer or the recycler. Removal of the mercury switches and replacement with ball bearing switches is relatively simple. The paperwork involved, the mechanic’s time, transport to recycling center, and the low payment of $1 per switch has done little to encourage recovery of the switches before shredding. Recyclers in Maine turned in 1,613 switches in 2003. This was less than 5 percent of the goal of 40 kg (approximately 1.2 flasks) of mercury. Solutions to the mercury switch problem in automobiles were addressed during a U.S. Environmental Protection Agency (EPA), Office of Solid Waste-sponsored State and Regional Roundtable—Mercury Automobile Switches, in Washington, D.C. on August 11, 2003. Therefore, because of the controversy surrounding responsibility for switch removal and to remove mercury from the recycling stream, the domestic automobile recycling industry has responded by considering recycling steel only from foreign vehicles. New Jersey has passed legislation that would require automakers to fund a State program for removing mercury switches from scrap vehicles. The goal of the program is to keep mercury from getting into the shredded scrap, and removal of the switch would entitle the dismantler or scrap yard to a $2 payment. The number of switches being recycled is increasing, although no data on the actual number of recycled domestic automobile convenience switches are available. Dental amalgamDental amalgam was introduced into the United States as a filling for decayed teeth in 1833 and was originally composed of mercury and silver. Modern amalgam contains mercury (50 percent), silver (34-38 percent), tin (12-14 percent), copper (1-2 percent), and zinc (0-1 percent). Approximately 30 t/yr of mercury is used for dental amalgam. A mercury amalgam filling may last from 2 to 20 years depending on the size of the filling. In Washington State, approximately 185 kilograms per year (kg/yr) of dental amalgam is recovered and recycled. Given that mercury comprises approximately one-half of the composition of dental amalgam, then approximately 90 kg/yr of mercury may be recovered from dental amalgam in Washington State alone. The use of amalgam is declining, and composite resin substitutes are available. The Watson-Burton bill (H.R. 1680, Mercury in Dental Filling Disclosure and Prohibition Act) seeks to prohibit the introduction of mercury for dental fillings into interstate commerce after 2008. Some public health organizations, however, require that dental amalgam be used. The American Dental Association (ADA) and State organizations recognize several use categories of amalgam and encourage recycling and use of separation devices. In addition, the dental profession sponsored a symposium for Federal and State officials that addressed policies related to increasing the use of mercury-free fillings, best management and recycling practices for amalgam, and operation and maintenance of amalgam separators. Dental amalgam has also been recycled to recover silver. No data on the amount of mercury that is recovered are available. The Office of Solid Waste met with amalgam producers, environmental service companies, recycling companies, and representatives of the ADA to advance the proper handling and recycling of dental amalgam waste that is generated at more than 100,000 dental offices in the United States. This collaborative effort, the National Partnership for Environmental Priorities Program, will promote responsible management of amalgam waste by use of a specific dental office collection device (a “gray bag”) to store dental waste until it is removed for recycling. The EPA and the ADA are encouraging voluntary participation in the “gray bag” recycling effort to track and prevent amalgam waste from being landfilled and to avoid legislation and mandatory adherence to amalgam collection and recycling. A Swedish Government agency proposed that amalgam fillings be removed from the dead before cremation to cut emissions of mercury. The agency calculated that three-quarters of Swedish citizens have amalgam fillings that could amount to 2.8 t of mercury, and given the 70-percent cremation rate, approximately 1.9 t/yr of mercury may be released through cremation. This mercury could then be made available for recycling. A study of mercury and the cremation process in the United Kingdom indicated that approximately 11 kg of mercury was released from one crematorium chimney in 1 year. Fluorescent LampsReclamation of mercury from spent fluorescent lamps and mercury-vapor lamps began in the United States in 1989, and the startup recycling rate ranged from 10 to 12 percent. That rate, however, increased to about 20 percent by the end of 2000. Of the 670 million lamps discarded each year, nearly 150 million are recycled; the business sector recycles approximately 27 percent, and only about 2 percent of residential lamps are recycled. The National Electrical Manufacturers Association (NEMA) indicates that fluorescent lamps sold in the United States in 1999 contained approximately 13 t of mercury, with each lamp containing from 10 to 20 milligrams of mercury. The recycling rate of approximately 20 percent implies that 80 percent of lamps ultimately become part of a landfill. Therefore, approximately 2.6 t of mercury may be recycled from fluorescent lamps, and the balance (approximately 10.4 t) is not recycled because the lamps may break in dumpsters or en route to the landfill, thereby instantaneously releasing the mercury into the environment. Elevated airborne levels of mercury exist in the vicinity of recently broken lamps, and because discarded lamps are likely to be broken during conventional waste handling, exposure of workers who handle these materials is of concern. When the lamps are properly handled, the mercury can be reclaimed and recycled and the risk to workers is reduced. For example, a 55-gallon drum (209 liters) that contains mercury-bearing phosphor powder from properly crushed lamps may contain approximately 1 tablespoon (15 milliliters or 0.0015 g) of mercury. AERC Recycling estimated that its U.S. recycling operations collect 10 t/yr of mercury from lamps, computer electronics, batteries, and dental amalgam. That material is sent on for further refinement. Approximately 9 million fluorescent tubes that contain approximately 0.18 t/yr of mercury is recycled from Government agencies in the Washington, DC, area. To respond to liability concerns, the recycling agency, USA Lights, issues a certificate to document the quantity of mercury-containing lamps that were received. Other lamp recyclers also issue an environmental statement of compliance to businesses that recycle their fluorescent lamps. Some fluorescent lamps now include an information panel that has the symbol for mercury “Hg” and a statement that the lamp contains mercury and should be disposed of in accordance with disposal laws. A Web site (www.lamprecycle.org) and a telephone number (1-800-435-4448) are provided for further information. The Association of Lighting and Mercury Recyclers (ALMR), NEMA, and the Solid Waste Association of North America (SWANA) are working with the EPA to increase the fluorescent lamp recycling rate through outreach and education. Their collaboration has resulted in an educational CD “Lamp Recycling Outreach Program,” which is aimed at anyone who handles spent lighting material or manages recycling and disposal decisions. Their goal is to increase the fluorescent lamp recycling rate to 40 percent by 2006 and 80 percent by 2009. Lab/MedicalMercury is used domestically in laboratories and in a number of medical devices, such as sphygmomanometers and thermometers (approximately 1,814 kg each) and manometers (approximately 350 kg). Sling psychrometers, which used two mercury thermometers to calculate relative humidity and were frequently broken, have now been replaced by digital instruments. Mercury is also used in gastrointestinal dilators. The Mercury Reduction and Disposal Act of 2001 (S.351) called for a ban on sales of mercury thermometers, established a grant to help consumers exchange mercury thermometers for digital ones, and required that the mercury collected from the thermometers (up to 17 t/yr) be kept out of commerce. Some environmental agencies have even offered to replace mercury thermometers with digital thermometers. Although data are not available, only a small percentage of this domestically produced mercury waste is estimated to have been recycled. Specialty steel dial thermometers used in boilers, food-processing equipment, industrial ovens, and milk coolers also use mercury. Domestic mercury recycling flow estimates become murky because other countries may send their mercury waste to the United States for processing. For example, 320 t of mercury-contaminated waste was sent from a plant in India to the United States for recycling. At the same time, India has become one of the world’s largest importers (approximately 550 t in 2002) of mercury. In India, mercury is widely used for batteries, chlorine-caustic soda production (23 plants use from 100 to 150 t/yr), fungicides, lamps and medical devices. In 2001, a shipment of mercury that was sent to India from a closed chlorine-caustic soda plant in the United States was denied entry by the Indian Government, and the mercury was unloaded at a port in Egypt to await return to the United States. More than one-half of end-of-life electronics, which include mercury-bearing computers, are shipped to Asia where the technology for recycling is limited. No data are available on the amount of mercury recycled from laboratories and medical devices. ThermostatsMercury-switch thermostats contain from one to four ampules of mercury, each of which contains approximately 2.7 g of mercury for a total mercury content that may range from 2.7 to 10.8 g of mercury per thermostat. According to the NEMA, more than 50,000,000 mercury switch thermostats are in service in the United States. Approximately 1.8 million mercury switch thermostats are brought out of service annually with only 1 to 5 percent of that total being recycled. Recycling efforts are hindered by the lower cost of mercury thermostats and their longevity (20-40) years compared with 10 years for the more-expensive, programmable, energy-saving, non-mercury thermostats. The use of mercury in thermostats is less than one-half of that used 3 to 4 years ago, and the 5 percent being recycled may increase as mercury thermostats are replaced with digital thermostats. Thermostat Recycling Corporation (TRC) announced that it has recovered approximately 29 flasks of mercury from 250,000 used mercury switch thermostats that were returned by heating, ventilation, and air-conditioning contractors since it began recovery of these devices and reclamation of mercury in 1998. In 2000, TRC recovered three flasks of mercury from 31,611 thermostats and in 2003, it collected eight flasks of mercury from 65,000 thermostats, which more than doubled the amount of mercury recovered in 2000. The Colorado Department of Public Health and TRC provide information on collection procedures for recycling these devices. Packaging, freight, certificates of recycling, and other instructions for returning small quantities of mercury-containing waste, such as thermostats or ballasts, for recycling are provided by Onyx Environmental Services. Chlorine-Caustic Soda IndustryThe preparation of chlorine-caustic soda has been an important use for mercury. The metal serves as a cathode in the electrolytic cell into which sodium chloride brine is introduced. An electric current is passed through the brine, chlorine gas is released at the anode, and sodium forms an amalgam with the anode metal mercury. Water is then added to the amalgam to remove the sodium, and the used mercury, or sludge, is recycled in-plant into the electrolytic cell. This reused mercury may also be called “home scrap,” and because it is recycled in-plant, it is not a part of the larger mercury recycling flow. Approximately 3,000 t of mercury is in use in the domestic chlorine-caustic soda industry that will ultimately have to be managed by recycling, sale, or storage as a result of the eventual closure of the plants. After closure, this mercury will enter the out-of-plant recycling flow as old scrap. In the United States, nine chlorine-caustic soda plants use mercury cell technology to produce chlorine and caustic soda. For example, approximately 48 t of mercury is routinely recycled on-site as home scrap at one plant in West Virginia. Closure of a mercury cell chlorine plant in Maine resulted in the disposition of more than 100 t of mercury. The U.S. Environmental Protection Agency 2000 Toxics Release Inventory indicated that approximately 65 t of mercury used in the chloralkali industry could not be accounted for. In another report, also by the EPA, an estimated 145 t/yr of mercury is consumed by the chloralkali industry. In its sixth annual report to the EPA, the Chlorine Institute indicated that 86 t of replacement mercury was purchased in 2000. This quantity is shown in Figure 2. Other references indicate that losses of mercury during the chlorine-caustic soda production process have declined from 200 grams of mercury per metric ton of chlorine output in the 1960s to only 0.2 gram of mercury per ton of chlorine produced today. This mercury may have vaporized, been released to the environment, or accumulated in pipes or plant equipment. In 1992, the EPA banned land disposal of high mercury-content sludge generated from the electrolytic production of chlorine-caustic soda. India’s 23 chloralkali plants use from 100 to 150 t/yr of replacement mercury, and brine sludge that contains as much as 65 percent mercury is lost as a contaminant. In South America, 13 chloralkali plants in South America use mercury, and no information on replacement mercury or treatment of the sludge is available. Other UsesArtisanal gold miningIn many placer gold operations in the United States, especially Alaska and California, in the 1800s, mercury was used to amalgamate the gold flakes in the sediment. This practice, which is also called artisanal, or small-scale, mining, continues as an important end-use of mercury in many parts of the world, such as, Ghana, Peru, Venezuela, and Vietnam. For example, mercury is provided by a vendor in Lima, Peru, for unspecified purposes in South America. It is possible that this mercury may be used in artisanal mining. Unfortunately, mercury for artisanal gold mining is rarely recycled and is released to the environment. At the Regional Awareness Raising Workshop on Mercury Pollution— A Global Problem That Needs To Be Addressed, which was held in Buenos Aires, Argentina, in September 2004, several papers were given that discussed the use of mercury for artisanal mining in Central America and South America. ConstructionMercury products used in buildings should be removed prior to demolition so that the mercury can be recycled. These products include flame sensors in gas ranges, flow meters, freezers, fungicidal rubber gym floors, lamps, switches in sump pumps, and water heaters. Removal of a gas meter, for example, recently resulted in a mercury spill that resulted in evacuation of the residents from a Washington, DC, home. Recognition of and instructions for removal and recycling of mercury products, such as flame sensors, probes, and sump pumps are now included in the training curriculum for HVAC professionals. ElectronicsComputer circuit boards, electrical switches, batteries, and fluorescent lamps for backlighting computer screens and panel displays also use mercury. The amount of mercury in a computer may vary between 50 milligrams (mg) and 45 g. Some of this mercury along with other toxic materials may be recycled. Trace amounts of mercury may be found in automobile headlights, liquid crystal displays (LCDs), and TV screens. The LCDs used in camcorders, cameras, fax machines, personal digital assistants, and projector televisions may contain up to 100 mg of mercury. No information is available on mercury recovered from these uses. Although recycling programs have been somewhat effective, mercury is included in the approximately 3.5 percent of “other” metals recovered from computers and electronics. Imports and exports with unspecified mercury contentThe U.S. Census Bureau publishes import and export statistics on elemental mercury of unspecified origin that may either be primary or recycled. A second trade category (Harmonized Tariff Schedule code 2843.90) includes “Inorganic or organic compounds of precious metals, whether or not chemically defined; amalgams of precious metals.” This category may contain unspecified amounts of mercury based on the metallurgical definition of amalgam as an alloy of mercury with any other metal. In 2000, the United States imported 89 t of this nonspecific “amalgam” material; 51 t came from Canada, and 22 t came from Japan. In 2000, the United States exported more than 1,400 t of this nonspecific material; 1,142 t went to Mexico, 89 t went to Brazil, and 81 t went to Canada. U.S. imports of several mercury-containing compounds, such as mercuric chloride, mercuric iodide, or mercurous chloride, from Asian, Latin American, and Mexican ports is provided by the Port Import Export Reporting Service. No information is available on the amount of mercury contained in these import/export categories. U.S. Government Mercury StockpileThe United States has maintained a mercury stockpile for strategic use. In the 1950s, 10,900 t of the Government’s mercury stockpile was tapped by the President for use at the Oak Ridge National Laboratory for the lithium extraction required for production of the hydrogen bomb. Today, however, Government stocks of mercury contain 4,436 t of mercury stockpiled at various locations around the country by the Defense Logistics Agency (2003, p. 4). In early 2004, the Agency indicated that the stockpiled mercury would be consolidated at one site. Discontinued and dissipative usesMercury batteries that contain zinc or cadmium with mercuric oxide have a good shelf life, high energy density, and reliable voltage were widely used in military applications, cameras, and watches. Owing to the development of other technologies and environmental concerns, mercury battery production was discontinued in the mid-1990s in the United States. Some countries, however, still manufacture inexpensive mercury “button” batteries for export. These have become a health risk in Peru because some children have eaten the shiny batteries and become blind. These types of batteries are imported for use in children’s toys in the United States. After use, these batteries are commonly put into trash that becomes part of a landfill, and the mercury contained in these imported batteries is rarely recycled. Mercury was also used in house paints to extend shelf life and to kill mold and mildew. After studies showed that exposure to hazardous mercury vapor may occur, its use for this purpose was discontinued in the United States. At one time, mercury and mercury compounds were used extensively as seed disinfectants. Now, however, the use of mercury or mercury compounds is not permitted on food crops in the United States. Mercury is used in a number of chemical compounds and historically may have been used in a variety of cleansers and soaps, contact lens solutions, disinfectants, eye and ear preparations, photographic preparations, and nasal sprays. Mercury compounds are used for antiseptics, such as merbromin and ammoniated mercury, or in preservatives, such as thimerosol. Mercury bromide and mercury acetic acid were used in the coating of a specialized paper and film for use in hospitals, newspaper publishing, and microfiche printers. These specialized uses for mercury were to be discontinued in 1995. Mercury used for these applications is not recycled. Figure 1 shows the quantity of old scrap generated and its flow—through processing and use. The volumes of each category of mercury-containing material have the potential for significant change from year to year; much of the data on the figure is estimated. This is because the use of mercury has been in decline, mercury is a low-volume commodity (approximately 200 t/yr went into fabrication in 2000); and mercury sales are not tracked. Estimated data are indicated by an “e” following the number in the figure and are discussed in the following
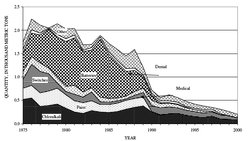
U.S. consumption of mercury for 1994 indicates the following approximate general use pattern: chlorine-caustic soda (42 percent), medical/lab use (26 percent), switches and electronics (24 percent), lamps (7 percent), and dental amalgam (6 percent). The overall decline and, in some cases, disuse of mercury-containing products make it very tenuous to project that use pattern for 2000. Similarly, industrial demand has declined from a high of 2,120 t in 1970 to approximately 200 t in 2000 as mercury substitutes become available and mercury available for recycling from mercury-containing (secondary) sources has declined (Fig. 2). Table 1 lists salient statistics from figures in the flow chart. Figure 2 shows how the various end-uses of mercury and, by implication, sources of secondary mercury for reclamation have declined since 1975; end-use distribution also declined for 2000. New scrap generatedNew scrap may be generated at plants that use mercury. For example, filling automobile switches, dental amalgam ampules, fluorescent lamps, medical devices and thermometers, and thermostat ampules may produce some spilled material, or new scrap, that is returned for recycling. No data are available on exact quantities of new scrap that are returned for recycling. Disposition of mercury scrap in landfillsIf mercury-containing products are not recycled, then these products and their contained mercury may be landfilled, incinerated, or otherwise released to the environment. In the United States, the amount of mercury in discarded products in municipal solid waste was approximately 640 t in 1989. As a result of declining use of mercury in fabricated products and increased restrictions on the use and disposal of mercury-containing products, that amount was projected to be approximately 160 t in 2000. Batteries and lamps made up 90 percent of the discarded products in 1989 and 80 percent of the discarded products in 2000. International mercury disposal and waste management are other important issues for the mercury recycling industry. For example, chlorine-caustic soda sludge is included on a list of waste types accepted at a dedicated placement site in Canada. Therefore, mercury-containing material may be sent to Canada to avoid an EPA ban on landfilling chlorine-caustic soda waste. No data are available on the amount of mercury contained in the sludge that may also be called “industrial waste” and that contains varying amounts of caustic soda, mercury, and water. The domestic mercury recycling industry is concerned and the environment is threatened by exports of mercury-containing waste that are shipped to landfills in Canada without retorting or reclamation of the contained mercury and unclear domestic regulations based on the size of landfilled debris, which is also referred to as the “debris loophole,” that permits unquantified amounts of secondary mercury that could be reclaimed and recycled to be landfilled in the United States with potential for release to the atmosphere or groundwater. As one example of this controversial problem, a health department in the Northwest United States estimated that more that 23 kg of mercury that could have been recycled from local dental offices was processed into a local landfill. The landfill manager indicated “the landfill does not knowingly accept mercury or hazardous wastes and the company relies on its customers to weed out mercury and has no detection equipment at the plant,” and a Department of Environmental Quality inspector for the region said, “I don’t doubt there is a lot of mercury going through the system and ending up in our landfill”. The approximately 95 t of mercury in mercury-containing waste that was landfilled in 2000 but could have been recycled is only an estimated minimum amount (Fig. 1). For comparison, in 1992, the EPA projected that approximately 160 t of mercury would be landfilled in 2000. Information on mercury in landfilled materials in the following section is condensed from a recently published analysis of the problem that was prepared for ALMR: Landfilling mercury-containing wastes may result in significant releases to the air and to groundwater. The Hazardous and Solid Waste Amendments of 1984 were added to the Resource Conservation and Recovery Act and required that waste be treated by the Best Demonstrated Available Technology (BDAT) prior to land disposal of the waste. For most highly contaminated mercury-containing waste, the U.S. Environmental Protection Agency considered BDAT to be mercury recovery using high temperature retorting. Treatment of these wastes by retorting is more expensive than burying the waste in a domestic landfill or exportation of the waste to Canada. Because of minimal or no treatment in Canada, there is a trend toward land disposal of mercury-containing waste in Canada rather than domestic reclamation and recycling. The rate of export of hazardous waste to Canada increased during the period 1995-2001 and the shipment of mercury-containing waste labeled as non-hazardous to Canada has also increased by an unquantifiable amount. Recommendations to increase recycling of mercury-containing waste destined for any landfill include: 1) use total mercury content for determining if the waste is hazardous; 2) ban landfilling of all mercury-containing lightbulbs; and 3) close the so-called “mercury-debris loophole,” which is a U.S. Environmental Protection Agency regulation that indicates that treatment standards for all mercury-containing debris in excess of 60 mm would be suspended. Mercury-containing hazardous waste has been so broadly defined that all mercury-containing waste found at landfill sites is classified as “debris,” and therefore, may be encapsulated and landfilled rather than recycled. In a review of 2001 shipping documents to only one of twenty landfills designated for hazardous waste, it was shown that the mercury waste was shipped as “debris” and in some cases as “high mercury debris.” The amount of “debris” was from one to 372 tons and the amount of contained mercury was unknown. The debris loophole, which permits landfilling of mercury-containing material without retorting, is a very serious recycling industry concern. An attorney for Mercury Waste Solutions indicated the following: The specified technology of high-temperature mercury recovery (retorting) is mandated for inorganic waste that is in the high-mercury subcategory (260 mg of Hg per kg or greater). Hazardous debris is debris that contains a hazardous waste such as mercury. For mercury-contaminated debris, treatment includes encapsulation regardless of mercury concentration and no mercury reclamation is required prior to landfilling. Mercury may also be sent to a landfill from a conditionally exempt small quantity generator (CESQG), an entity that produces less than 100 kg of hazardous waste per month and may include mercury-containing products. Waste from a CESQG may be delivered to a hazardous waste landfill, a municipal waste landfill, or delivered to a recycling or universal waste site. CESQRs are exempt from other Federal hazardous waste requirements." Tracking U.S. mercury imports and exports is also a goal of the Canadian Commission for Environmental Cooperation. In 1997, the Governments of Canada, Mexico, and the United States committed to the North American Regional Action Plan on Mercury. Objectives of this collaboration include the identification and discussion of U.S. methodologies and processes for tracking imports and exports of mercury used in manufactured goods; the identification of U.S. reporting mechanisms used to track the ultimate fate of mercury-containing waste, particularly waste transported across national boundaries for storage, handling, processing, disposal, or long-term containment; and recommendations to improve international tracking and reporting systems. Although mercury products may technically be recycled, mercury cannot be recycled economically from most consumer products. Recycling reduces the liability associated with the handling of hazardous mercury-containing waste. Even so, many of these products may become part of a landfill. Regulations aimed at reclaiming mercury-containing waste allow most of that waste to be landfilled as hazardous waste. Disposition of mercury scrap and recycling is affected by the lack of information about products that contain mercury, the lack of information about proper disposal methods for these products, and, especially, the lack of consumer awareness about the health hazards and toxicity associated with mercury. For example, mercury stolen from a Washington, DC, high school laboratory was splashed around the school. This resulted in closure of the school for several days as a hazardous materials team cleaned up the spill . A cereal company has agreed to stop distributing products that contain promotional toys that use mercury batteries and have agreed to provide postage-paid return envelopes to customers who received any of the 17 million promotional toys. Collection and recycling of automobile switches are hampered by the question of who is responsible for removing and recycling the mercury-containing switches—automobile manufacturers, automobile recyclers, or consumers. A Federal judge has recently upheld a Maine law that requires automakers to pay for removal of mercury switches, and a proposed Massachusetts law will allow motorists to have the switches replaced for free while the vehicles are still in service. Only about 20 percent of fluorescent lamps are recycled, which leaves the remaining 80 percent destined for disposal at landfills with a high potential for breakage during handling and subsequent mercury release. Data on the percentages of mercury products that ultimately become part of landfills are estimated to be similarly high; exact figures, however, are not available. Old scrap recycling efficiencyMaking an estimate of domestic old scrap recycling efficiency, excluding the approximate 3,000 t of mercury held and recycled as home-scrap in the chlorine-caustic soda industry, is not easy. More mercury-containing products may be recycled given the combined effects of national regulations aimed at reclaiming hazardous waste, lack of domestic mercury mining, increased demand, heightened environmental concern, and a recent rise in the price for mercury to $650 per flask. This rise in price is caused by the decline in the fabrication of mercury-containing products since 1980, which has limited the amount of secondary mercury that may be recycled, thus forcing an increased dependence on recovery and processing of byproduct mercury. On the basis of estimated old scrap generated and quantity of mercury consumed, the recycling efficiency was approximately 62 percent in 2000. Infrastructure and processingMercury from most mercury-containing products may be recycled. Individual segments of the industry, such as manufacturers and recyclers, vary in their approach to recycling. Some fluorescent lamp manufacturers now label their products and provide consumers with recycling information through a toll-free number and a Web site. Collection and shipping instructions are included on Web sites. For example, AERC Recycling provides packaging and shipping guidelines for computers, electronic scrap, fluorescent lamps, and other mercury-containing products. Onyx Environmental Services will coordinate proper packaging (OnyxPak) and transport of the used fluorescent lamps, ballasts, thermostats, and dental waste. Mercury Recyclers and the Ohio Office of Pollution Prevention list forms of mercury accepted, hazardous waste manifest considerations, mercury prices, packaging requirements, shipping costs, and other requirements. ALMR provides disposal information on a number of mercury-containing products. Similarly, the U.S. Environmental Protection Agency provides information on where mercury-containing products are found and what should be done to recycle these products. General information on regulations and lamp recycling is provided by Fluorescent Lights and Mercury. Scrap material is treated much the same as mercury ores to recover the mercury. In general, mercury-containing products are broken or crushed in sealed vessels, and then the feed is heated (mercury boils at 357 °C, or 675 °F) in a retort to volatize the mercury, which is then condensed and filtered to a high-purity metal. Drum crushers may be used in the initial treatment of fluorescent lamps; regulations, however, on this procedure vary by State. In 1990, the EPA’s Land Disposal Restrictions indicated that hazardous wastes that contained 260 milligrams per kilogram (mg/kg) or more of mercury must be retorted before being sent to a landfill. Temperatures in the retort range from 425 °C to 540 °C, and technical details of the retorts and recovery processes are given in Washburn and Hill. Other special-use recovery technologies include acid leaching and specific processes for the removal of mercury-contaminated soil near chloralkali plants or Superfund sites. OutlookThrough reclamation and recycling of secondary and byproduct mercury, the mercury recycling business is important in reducing the liability of users of mercury-containing products and, at the same time, reducing mercury releases to the environment from mercury-containing products, such as fluorescent lamps, that have been landfilled. Even though overall use of mercury in the United States is declining, mercury-containing products are still available, and elemental mercury is an integral part of the chlorine-caustic soda industry. Human health and environmental concerns, however, are mainstream issues influencing how mercury is used domestically. The recycling rate for mercury may be improved by the following:
Further reading
|