Heat transfer

An object’s kinetic energy can be classified as internal or external. For example, a falling coin has a certain external kinetic energy that is related to its overall mass and to its velocity as it falls. The coin is also composed of particles that, like all particles, are moving in a random way, independent of the overall motion (or position) of the coin. The particles in the coin are constantly moving, colliding, changing direction, and changing their velocities. The energy associated with this internal motion is internal kinetic energy (Figure 1).
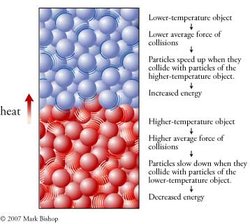
The internal kinetic energy of an object can be increased by putting it in contact with another object at a higher temperature. Temperature is proportional the average internal kinetic energy of an object, so higher temperature means a greater average internal energy for the particles within the object. The particles in a higher-temperature object collide with other particles with greater average force than the particles of a lower-temperature object. Thus collisions between the particles of two objects at different temperatures cause the particles of the lower-temperature object to speed up, increasing the object’s energy, and cause the particles of the higher-temperature object to slow down, decreasing this object’s energy. In this way, energy is transferred from the higher-temperature object to the lower-temperature object. We call energy that is transferred in this way heat. The energy that is transferred through an object, as from the bottom of a cooking pan to its handle, is also called heat. Heat is the energy that is transferred from a region of higher temperature to a region of lower temperature as a consequence of the collisions of particles (Figure 2).
The internal kinetic energy of an object, and therefore its temperature, can be increased in three general ways. The first way is to rub, compress, or distort the object. For example, after a good snowball fight, you can warm your hands by rubbing them together. Likewise, if you beat on metal with a hammer, it will get hot. The second way to increase the internal kinetic energy of an object is to put it in contact with another object at a higher temperature. This process by which heat is transferred by direct contact between an object at a higher temperature to one at a lower temperature is often called conduction. The term convection is often used to describe heat transfer that ccurs between a object and a fluid flowing across it. Convection is, in essence, a type of conduction. The third way an object’s internal kinetic energy and temperature are increased is by exposure to radiant energy, such as the energy coming from the sun. The radiant energy is converted to kinetic energy of the particles in the object. This is why we get hot in the sun.
Further Reading
- The initial version of this article was an exerpt from the preparatory chemistry text An Introduction to Chemistry by Mark Bishop.