Ecosystems and Human Well-Being: Volume 1: Current State and Trends: Marine Systems
This is Chapter 18 of the Millenium Ecosystem Assessment report Ecosystems and Human Well-Being: Volume 1: Current State and Trends
Coordinating Lead Authors: Daniel Pauly, Jacqueline Alder
Lead Authors: Andy Bakun, Sherry Heileman, Karl-Hermann Kock, Pamela Mace, William Perrin, Kostas Stergiou, Ussif Rashid Sumaila, Marjo Vierros, Katia Freire, Yvonne Sadovy
Contributing Authors: Villy Christensen, Kristin Kaschner, Maria-Lourdes Palomares, Peter Tyedmers, Colette Wabnitz, Reg Watson, Boris Worm
Review Editors: Joseph Baker, Patricia Moreno Casasola, Ariel Lugo, Avelino Sua´rez Rodr?´guez, Lingzis Dan, Ling Tang
Main Messages
All oceans (Ocean) are affected by humans to various degrees, with overfishing having the most widespread and the dominant direct impact on food provisioning services, which will affect future generations. Areas beyond the 50 meters depth are mainly affected directly by fishing and indirectly by pollution. Fish are also directly affected by coastal pollution and degradation when their life cycle takes them into coastal habitats. Recent studies have demonstrated that global fisheries (Fisheries and aquaculture) landings peaked in the late 1980s and are now declining despite increasing fishing effort, with little evidence that this trend is reversing under current practices. Fishing pressure is so strong in some marine systems that the biomass of some targeted species, especially larger high-value fish and those caught incidentally (the ‘‘bycatch’’), has been reduced to one tenth or less of the level that existed prior to the onset of industrial fishing. In addition, the average trophic level of global landings is declining, which implies that we are increasingly relying on fish that originate from the lower part of marine food webs.
Industrial fleets are fishing with greater efficiency, further offshore, and in deeper waters to meet the global demand for fish. Until a few decades ago, depth and distance from coasts protected much of the deep ocean fauna from the effect of fishing. However, recent large investments in fishing capacity and navigation aids have led to fleets that now cover the world’s ocean, including polar and deep, low-productivity areas, where catches are affecting easily depleted populations of long-lived species. The biomass of large pelagic fish in these areas taken by longlines, purse seines, and drift nets has also plummeted. Studies on available data have shown that deep-sea fisheries that collapsed in the 1970s have not recovered.
Overfishing has negative impacts on marine biodiversity (Global marine biodiversity trends). The lowered biomasses and fragmented habitats resulting from the impacts of fishing have led to local extinctions, especially among large, long-lived, slow-growing species with narrow geographical ranges. In addition, the ability of the component ecosystems and their embedded species to withstand stresses resulting from climate change (Climate Change (collection)) and other human impacts will be reduced, though direct demonstration of this effect may not be evident in many systems for some decades.
Destructive fishing practices have long-term impacts on marine habitats. Destructive practices such as trawling, dynamiting, and dredging change the structure of marine ecosystems, with consequential changes in their capacity to provide services, such as food provisioning and income generation. Longterm losses in species and habitats through destructive fishing ultimately reduce the biodiversity of these affected systems, resulting in a further loss of services such as coastal protection. Some systems may recover and improve the availability of some services and products fairly quickly; other more vulnerable systems, such as cold-water corals and seamounts, may take hundreds of years to recover.
The implementation of no-take marine reserves combined with other interventions, such as controls on fishing capacity, would be a more proactive response to fisheries management than current reactive approaches. Marine reserves can contribute to better fisheries management—helping to rebuild stocks through enhanced recruitment and spill-over effects, maintaining biodiversity, buffering marine systems from human disturbances, and maintaining the ecosystems that fisheries rely on.
Aquaculture is not a solution to the problem of declining wild-capture fisheries. Good governance and effective management of wild-capture fishing are likely to be more successful approaches. Farmed species such as salmon and tuna, which use fishmeal, may in fact contribute to the problem since much of the fishmeal and oil currently used in the aquaculture industry is derived from wild-caught small pelagic fish. In some countries, such as Chile, small pelagic fish that were once a source of cheap protein for people are now largely diverted for fishmeal.
The supply of wild marine fish as a cheap source of protein for many countries is declining. Per capita fish consumption in developing countries (excluding China) has declined from 9.4 kilograms per person in 1985 to 9.2 kilograms in 1997. In some areas, fish prices for consumers have increased faster than the cost of living. Fish products are heavily traded, and approximately 50% of fish exports are from developing countries. Exports from developing countries and the Southern Hemisphere presently offset much of the demand shortfall in European, North American, and East Asian markets.
The proposed future uses of marine systems pose significant policy challenges. Ocean ranching of marine organisms, bioprospecting, seabed mining, and carbon sequestration in deep ocean waters are foreseeable uses of marine systems. However, the potential impacts of these activities are not well known. In some cases no or only limited field studies have been conducted to test the theoretical basis for the activity. Policies will need to deal with the uncertainty of potential impacts and the limited understanding of marine biodiversity (Global marine biodiversity trends). National and regional ocean policies that incorporate zoning for various uses within an integrated ecosystem-based management framework are likely to be needed. Such policies might include marine protected areas that can contribute to the restoration of species and habitats and thus form part of a precautionary strategy for guarding against management errors.
18.1 Introduction
Most of Earth—70.8%, or 362 million square kilometers—is covered by oceans and major seas. Marine systems are highly dynamic and tightly connected through a network of surface and deepwater currents. The properties of the water generate different density layers, thermoclines, and gradients of light penetration in marine systems, which result in productivity varying vertically. Tides, currents, and upwellings break this stratification and, by forcing the mixing of water layers, enhance primary production.
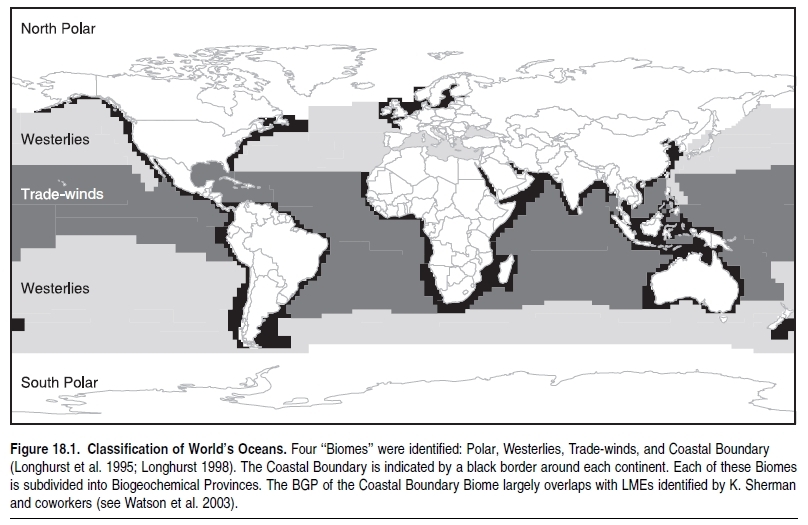
One widely accepted classification divides marine systems into four biomes (Longhurst et al. 1995; Longhurst 1998): the coastal boundary zone, trade-winds, westerlies, and polar. (See Figure 18.1.) These biomes are subdivided into a total of 57 biogeochemical provinces with distinct seasonal patterns of surface nutrient enrichment, which determine primary production levels and, ultimately, fisheries yield. The provinces of the coastal boundary zone biome largely overlap with the large marine ecosystems of K. Sherman and collaborators (see Watson et al. 2003), and hence those are implicitly included here. For practical reasons, we also refer to the U.N. Food and Agriculture Organization’s classification that has been used to report on global fisheries statistics since 1950 and that divides the world’s oceans up into 18 FAO statistical areas (FAO 1981).
The coastal boundary zone that surrounds the continents is the most productive part of the world ocean, yielding about 90% of marine fisheries catches. Overall, coastal and marine fisheries landings averaged 82.4 million tons per year during 1991–2000, with a declining trend now largely attributed to overfishing. The other three biomes are less productive, and their deep waters are exploited mainly for their large pelagic fish. The four biomes are described in detail in the next section.
In this assessment, the marine system is defined as the marine waters from the low-water mark to the high seas that support marine capture fisheries and deepwater ( 50 meters) habitats.This definition spatially overlaps with coastal systems, which are bounded inland by land-based influences within 100 kilometers or 100-meters elevation (whichever is closer to the sea) and seaward by the 50-meter depth contour. Chapter 19 (Ecosystems and Human Well-Being: Volume 1: Current State and Trends: Marine Systems) focuses on coastal habitats and coastal communities, however. It does not overlap conceptually with this chapter, which focuses on the condition and trends of fisheries resources in marine ecosystems for the following reasons:
- Living marine resources and their associated ecosystems outside of coastal areas (as defined by the MA), which maintain the food provisioning services of marine systems, have been affected over the last 50 years mostly by fishing.
- Our level of understanding of fisheries and the availability of information needed to assess the impact of fisheries are much better than for other human activities in marine systems. However, studies on biodiversity changes in marine systems are lagging behind our understanding of fisheries systems or terrestrial biodiversity changes. And overall, our understanding of long-term impacts and their interactions with other activities (current and future) is very limited.
- [[Chapter 19 (Ecosystems and Human Well-Being: Volume 1: Current State and Trends: Marine Systems)]2] describes the condition and trends of marine habitats and significant marine animals from the high-water mark to the 50-meter bathymetric line. Thus it discusses in detail the condition and trends of shallow inshore coastal habitats such as coral reef (Coral reefs (collection)) (Coral reefs (collection))s (Coral reefs (collection)), mangroves (Mangrove ecology), and seagrasses, as well as important fauna such as seabirds, turtles, and marine mammals. Since most human uses of marine systems (tourism, gas and oil extractions, and so on) occur in the coast, they are discussed in detail there. On the other hand, the impact of human use, especially fishing, on deeper-water systems such as shelves, slopes, seamounts, and so on are discussed in this chapter.
- Chapter 4 (Ecosystems and Human Well-Being: Volume 1: Current State and Trends: Marine Systems), on biodiversity, includes many non-fisheries aspects of marine biodiversity (Global marine biodiversity trends) not covered here. Chapter 12 (Ecosystems and Human Well-Being: Volume 1: Current State and Trends: Marine Systems), on nutrient cycling, discusses the cycling of carbon, nitrogen, and phosphorus and the changes in these cycles in marine systems. And Chapter 13 (Ecosystems and Human Well-Being: Volume 1: Current State and Trends: Marine Systems), on air quality and climate, highlights possible changes, including acidification, carbon sequestration, and fluxes in marine systems, over the short term.
Nevertheless, this chapter touches on various aspects of marine ecosystems such as marine biodiversity as they relate to fisheries and deepwater habitats, and some activities such as tourism and transportation are also mentioned. But there is currently insufficient information available to assess which activities have relatively more impact than others in marine systems.
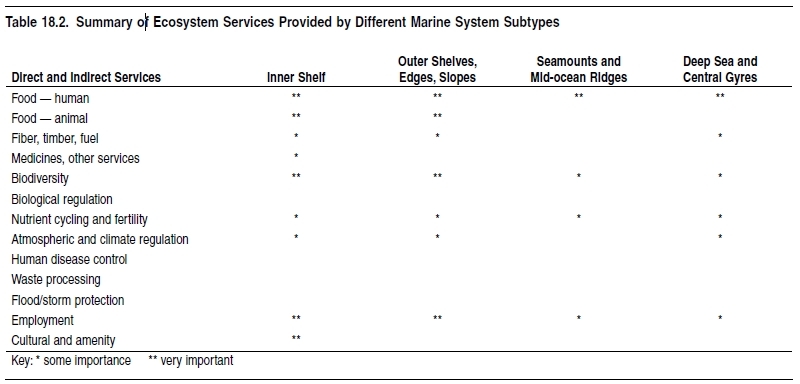
Marine ecosystems are diverse—some are highly productive, and all are important ecologically at the global scale and highly valuable to humankind. The major ecosystem services (as described in Chapter 1 (Ecosystems and Human Well-Being: Volume 1: Current State and Trends: Marine Systems)) derived from marine ecosystems are summarized in Table 18.1.
Marine systems play significant roles in climate regulation, the freshwater cycle, food provisioning, biodiversity maintenance, energy, and cultural services, including recreation and tourism. They are also an important source of economic (Economics of fisheries) benefits, with capture fisheries alone worth approximately $81 billion in 2000 (FAO 2002); aquaculture worth $57 billion in 2000 (FAO 2002); offshore gas and oil, $132 billion in 1995; marine tourism, much of it in the coast, $161 billion in 1995; and trade and shipping, $155 billion in 1995 (McGinn 1999). There are approximately 15 million fishers employed aboard decked and undecked fishing vessels in the marine capture fisheries sector. About 90% of these fishers work on vessels less than 24 meters in length (FAO n.d.).
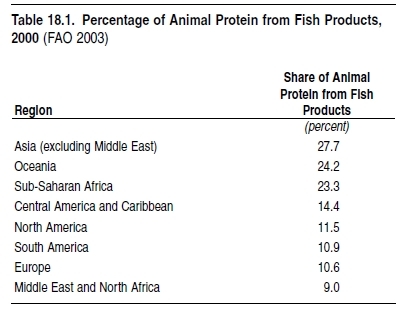
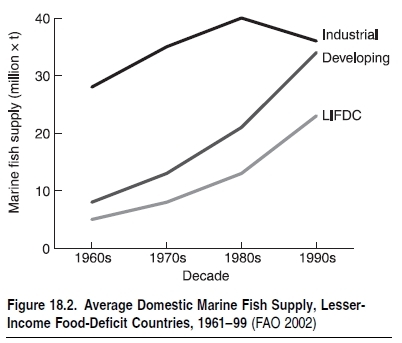
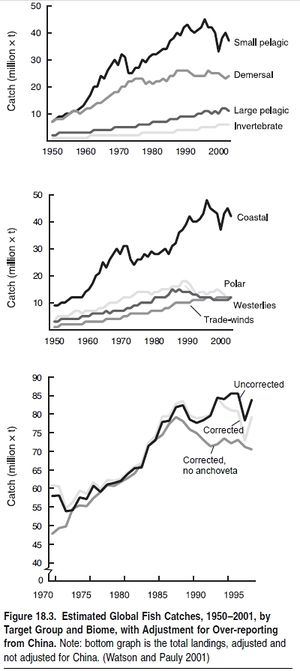
More than a billion people rely on fish as their main or sole source of animal protein, especially in developing countries. (See Table 18.2.) Demand for food fish and various other products from the sea is driven by population growth, human migratio toward coastal areas, and rising incomes that increase demand for luxury seafood. Detailed data on fisheries catches—that is, food provisioning, the major ecosystem service considered here—are available since 1950 for (groups of ) species for all FAO areas and maritime countries of the world. (See www.fao.org for tabular statistics and www.seaaroundus.org for spatially disaggregated statistics.) These show that catches increased more rapidly than the human population through the 1950s and 1960s, leading to an increase in available seafood. (See Figure 18.2.) This period also saw the depletion of many local stocks, but this was masked by the global increase of landings. The first fisheries collapse with global impact on prices of fishmeal and its substitutes was the Peruvian anchoveta, in 1971/72, which fell from an official catch of 12 million tons annually in the 1972–73 season (in reality, probably 16 million tons annually; see Castillo and Mendo 1987) to 2 million tons in 1973 (Tsukayama and Palomares 1987), ushering in two decades of slow growth and then stagnation in global fish catches. (See Figure 18.3.)18.2 Condition and Trends of Marine Fisheries Systems
18.2.1 Global Trends
The mid-twentieth century saw the rapid expansion of fishing fleets throughout the world and an increase in the volume of fish landed. These trends continued until the 1980s, when global marine landings reached slightly over 80 million tons per year; then they either stagnated (China included; FAO 2002) or began to slowly decline (Watson and Pauly 2001). However, regional landings peaked at different times throughout the world, which in part masked the decline of many fisheries.
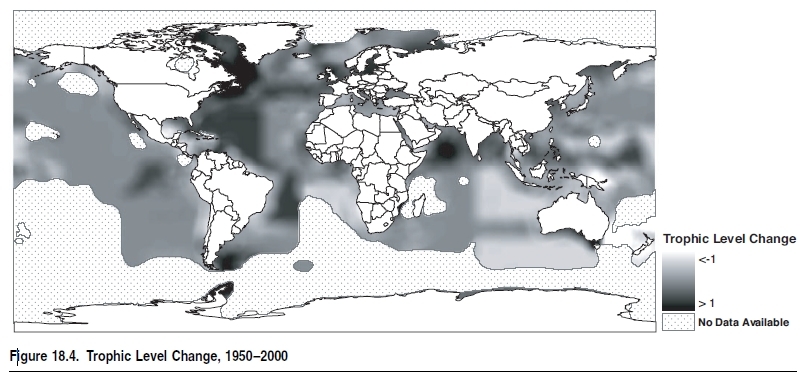
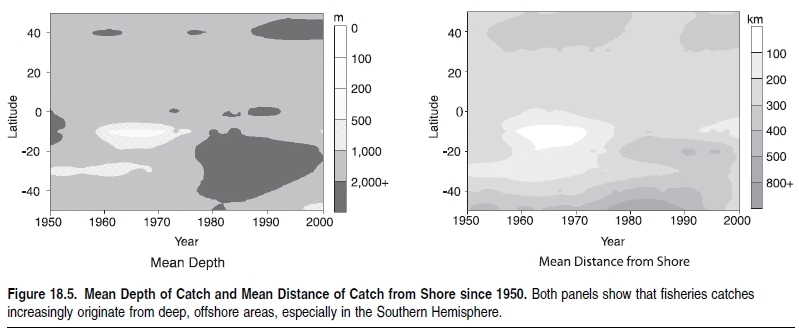
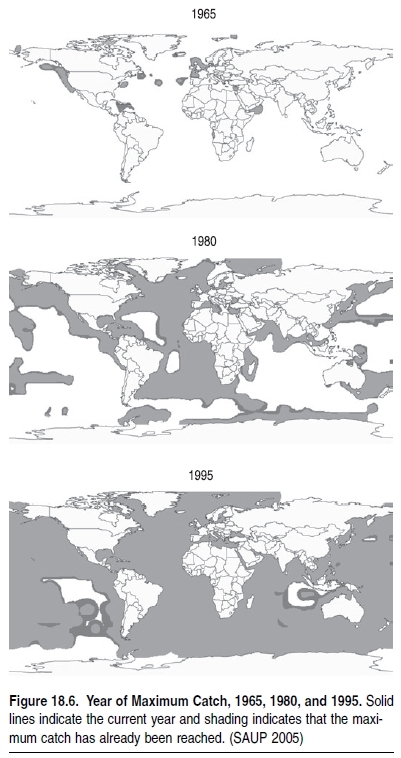
Indeed, the world’s demand for food and animal feed over the last 50 years has resulted in such strong fishing pressure that the biomass of some targeted species, such as the larger, higher-valued species and those caught incidentally (the ‘‘bycatch’’), has been reduced over much of the world by a factor of 10 relative to levels prior to the onset of industrial fishing (Christensen et al. 2003; Myers and Worm 2003). In addition, with fleets now targeting the more abundant fish at lower trophic levels (see Figure 18.4), it would be expected that global catches should be increasing rather than stagnating or decreasing, as is actually occurring. Indeed, this by itself indicates the extent that fishing has affected marine ecosystems.Changes in trophic levels of global and regional catches are considered a better reflection of trends in fisheries than the proportion of fish stocks that are reported as depleted, overexploited, fully exploited, and moderately exploited (FAO 2002). The FAO analysis lists the status of commercially important stocks where there is sufficient information. While the information presented is simple and many people use it to reflect the state of fisheries globally, they have the potential to provide an overoptimistic estimate of the state of fisheries. First, the figures presented only consider stocks currently exploited and exclude those that were fished either to extinction or abandoned over the last 50–100 years. Second, the reporting is based on over 1,500 stocks, with an assessed ‘‘stock’’ actually representing species distributed over large areas—that is, the aggregate of many stocks that are at varying states of exploitation. Moreover, the ‘‘stocks’’ presented do not represent the thousands of stocks that are fished by small-scale fishers that are not assessed or included in official statistics. For example, there are thousands of coral reef (Coral reefs (collection)) (Coral reefs (collection)) fish stocks that are fished by small-scale fishers in areas such as Indonesia and the Philippines, which are severely overfished but not a part of the FAO global analysis.Until a few decades ago, depth and distance from coasts protected much of the deep-ocean fauna from the effect of fishing (Figure 18.5). However, fleets now fish further offshore and in deeper water with greater precision and efficiency, compromising areas that acted as refuges for the spawning of many species of commercial interest to both industrial and artisanal fleets (Kulka et al. 1995; Pauly et al. 2003). (See Figure 18.6.) Investments in the development of fishing capacity have led to fleets that cover the entire world’s oceans, including polar and deep-sea areas and the low-productivity central gyres of the oceans. Trawl catches particularly target easily depleted accumulations of long-lived species, and the biomass of large pelagic fish has also plummeted (Worm and Myers 2003).
Not only are once inaccessible areas of the ocean increasingly being fished, they are also increasingly exploited for other ecosystem services. The marine realm is seen by many as the next frontier for economic development, especially for gas and oil and other energy sources (wind, gas hydrates, and currents), seabed mining (such as polymetallic nodules), bioprospecting, ocean dumping, aquaculture, and carbon sequestration. Worm et al. (2003) have identified pelagic ‘‘hotspots’’ of biodiversity (see Figure 18.7 in Appendix A), while Bryant et al. (1998) identified key coral reef areas. These and other hotspots, which may play a key role in supporting ecosystem services such as biodiversity, will be negatively affected by these developments unless they are appropriately managed.
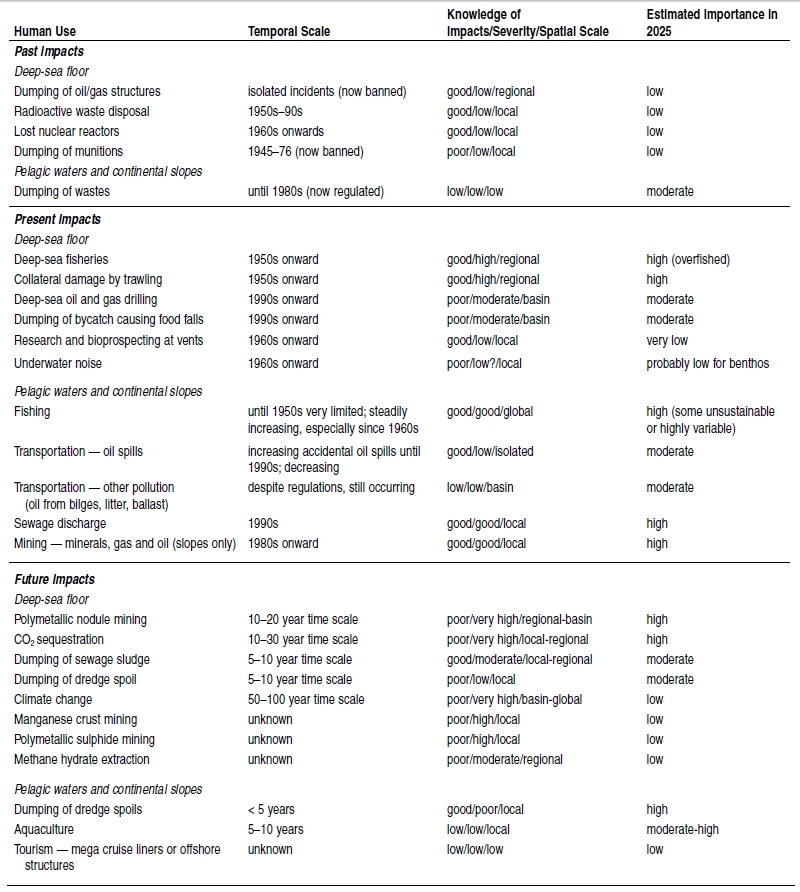
The gas and oil industry is worth more than $132 billion annually, and the potential for further development is considered high (McGinn 1999). Current levels of development in the deeper ocean environments are low, but future rises in the price of carbon-based fuels could make the extraction of crude oil and gas further offshore financially feasible.Mining in shallow offshore coastal areas for gold, diamonds, and tin is already under way and there is little doubt that the technology can be developed for deeper mining for a range of minerals, including manganese nodules, cobalt, and polymetallic sulfides, given appropriate economic incentives (Wiltshire 2001). It is assumed that institutional constraints in the future may ensure that activities in these deep-sea environments are conducted in a sustainable way and with minimal impact. This raises a number of questions regarding the nature and scale of the impacts on the little-known deep-ocean habitats. Mineral extraction may include ship- or platform-based processing of the extracted product, which has the potential to pollute the adjacent ecosystems. While bioprospecting does not have the magnitude of physical impacts that oil and mineral extraction does, there is nevertheless the potential for overexploitation (see Chapter 10 (Ecosystems and Human Well-Being: Volume 1: Current State and Trends: Marine Systems)), resulting in impacts similar to those described for overfishing.Ocean biomes are also strongly affected by humans. While the coastal biome is heavily affected by coastal development and landbased pollution sources (see [[Chapter 19 (Ecosystems and Human Well-Being: Volume 1: Current State and Trends: Marine Systems)]3]), the three other biomes have been affected by a variety of actions such as oil spills, overhunting of marine mammals, seabird mortalities, and ocean dumping of waste. (See Tables 18.3 and 18.4.) For instance, the estimated 313,000 containers of low-intermediate emission radioactive waste dumped in the Atlantic and Pacific Oceans since the 1970s pose a significant threat to deep-sea ecosystems should the containers leak (Glover and Smith 2003), which seems likely over the long term. Other examples include seabird populations that have been seriously affected by fishing and oil pollution, such as the estimated 14,000 seabirds killed each year by the Alaskan longline groundfish fishery between 1993 and 1997 (Stehn et al. 2001) and the chronic pollution along the coast of Chubut (Argentina) that has significantly increased Magellanic penguin (Spheniscus megellanicus) mortality (Gandini et al. 1994).
Knowledge of the effects of persistent organic and inorganic pollutants on marine fauna, including reproductive effects, is lim- ited, and we know even less about how these pollutants interact with fisheries impacts. Similarly, non-fishery factors and their impacts on habitats, primary productivity, and other ecosystem features are often described in the literature. However, their joint and cumulative effect on ecosystems is usually not assessed, limiting comparative analyses. Other human activities, as well as climate (due to natural variation and anthropogenic sources), influence marine systems, but their effects cannot usually be clearly separated from the impact of fishing. However, this should not detract from the urgent need to implement sustainable fisheries practices.
18.2.2 Coastal Boundary Zone Biome
The coastal boundary zone biome (10.5% of the world ocean) consists of the continental shelves (0–200 meters) and the adjacent slopes—this is, from the coastlines to the oceanographic front usually found along the shelf edges (Longhurst 1998). The 64 large marine ecosystems listed in Sherman and Duda (1999), which serves as a conceptual framework for an increasing number of multisectoral projects, largely match the biogeochemical provinces of Longhurst’s coastal boundary biome and hence are implicitly considered here.This biome fully includes the coastal systems (0–50 meters) covered in [[Chapter 19 (Ecosystems and Human Well-Being: Volume 1: Current State and Trends: Marine Systems)]4], the outer shelves (50–200 meters), and most of the continental slopes (200–1,000 meters). The coastal component of this biome is the first to have been accessed by fisheries, and it provides the bulk (53% in 2001; R. Watson, Sea Around Us Project, unpublished data) of the world’s marine fisheries catches. The major processes that lead to ecosystem services such as food provisioning and biodiversity from this biome are described here.Marine food webs are based largely on primary production by microscopic algae, the phytoplankton. This production occurs in the lighted, upper layers of the ocean, especially in the coastal zone, and is intensified by processes that lift nutrient-laden water from deeper layers. Most of this production is then either grazed by herbivorous zooplankton (mainly copepods) or falls to the sea bottom in form of detritus aggregates known as marine snow, which is formed of decomposed phytoplankton and zooplankton as well as the feces of the zooplankton attacked by bacteria while on the way down and consumed by benthic organisms upon reaching the sea floor. Little marine snow reaches the bottom of tropical seas due to, among other things, the higher metabolic rates of bacteria in warm waters. Hence, there is less benthos and fewer ground fish to catch in the deeper reaches of tropical seas than in otherwise comparable temperate or polar seas and upwelling systems. This creates a limit to the expansion of deep-sea benthic fisheries in tropical areas (Longhurst and Pauly 1987).
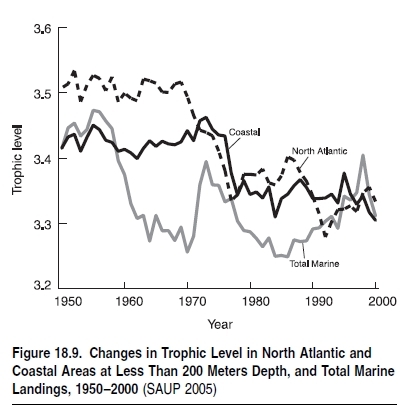
The coastal boundary biome is the most significant source of marine fish landed globally, and it also bears many of the impacts of fishing on ecosystems and of other human activities. Most depleted stocks are in this biome. In the North Atlantic, this has resulted in substantial marine biomass declines over the last 100 years (see Figure 18.8 in Appendix A) as well as the mean trophic level of the catch (see Figure 18.9) over the last 50 years. The majority of bottom:trawling fleets operate in this zone, affecting large areas of the seabed on a continual basis while catching both target and nontarget species. Holmes (1997) suggested that trawling destroyed seabed habitat and this contributed to fish declines in heavily trawled areas. A century of trawling in the North Sea has reshaped part of the seabed and changed the structure of the ecosystem (Malakoff 1998). Areas of the greatest decline in landings are in the coastal boundary biome.
Fishing pressure in this biome is not just attributed to excessively large industrial fleets but also to small-scale or recreational fishers, whose landings have a minor impact when viewed individually but who collectively can significantly deplete local resources, as described later. Coastal habitats such as coral reef (Coral reefs (collection)) (Coral reefs (collection))s (Coral reefs (collection)) and similar biogenic bottom structures (for example, soft corals and sponge beds) are degraded where destructive fishing methods such as explosives, poisoning, and trawling are used by small-scale fishers (Cesar et al. 2003). Such fishing practices have particular impacts on coral reef habitats and the ability of damaged reefs to recover.
Coral reef fisheries are overexploited in many reef systems around the world (Christensen and Pauly 2001; Jameson et al. 1998). Although many fisheries have become unsustainable due to the scale of high-technology improvements to boats and fishing gear, even small changes of technology can shift the balance toward unsustainability. In Pacific Islands, for instance, spearfishers’ catch of large humphead wrasse (Cheilinus undulates) used to be limited due to their reliance on snorkeling gear. However, scuba diving equipment has recently given fishers access to wider areas of reef and let them use other methods such as cyanide and dynamite both day and night, decimating humphead wrasse as well as populations of other large fish that are sold to the live fish market (Birkeland and Friedlander 2002).
The trading of fish sourced in the coastal boundary biome has undermined food security (Marine biodiversity and food security) in coastal communities of the developing world. The demand for fish in local, regional, or international markets can, though increased prices, promote overfishing when demand from a luxury market largely exceeds the supply and fisheries management is ineffective. Eight of the top 40 fooddeficient countries are also major fish producers and exporters (Kurien 1998). Much of the fish from the coastal boundary biome is exported to industrial countries, often to service the national debt of developing nations. The export of captured marine fish from developing countries has removed a cheap source of protein from their people in some cases. Senegal, for instance, which is a significant exporter of marine products, also has a protein deficit among its rural population because the growth of export-oriented fisheries disrupted domestic supplies of cheap, small pelagic fish (UNEP 2002a).Table 18.3. Summary of Human Disturbances at the Deep-sea Floor, in Pelagic Waters, and on Continental Slopes (Deep-sea floor from Glover and Smith 2003) In contemporary literature on fisheries economics, it is accepted that once fisheries cease to be open access (by instituting some form of property rights), they can be managed sustainably to ensure the holder of quotas the maximum discounted eco- nomic rent, which is the highest present value of the sum of all future flows of resource rent from a given fishery (Hannesson 2000; Arnason and Gissurarson 1999). However, many authors have challenged this view because whether the stock is managed sustainably will also depend on a number of other factors, including the price-cost ratio of landing a unit weight of fish and the discount rate applied to calculate the discounted rent. If both this ratio and the discount rate are very high, it may well be economically rational for the quota holder to deplete the fish stocks. In fact, it will be optimal in a strictly economic sense, as doing so may provide the maximum economic rent from a resource (Clark 1973; Heal 1998; Sumaila 2001; Sumaila and Walters 2004). Current economic models for fisheries, including those based on property rights, need to better accommodate underlying biological constraints.18.2.3 Trade-winds BiomeThe trade-winds biome (covering 38.5% of the world ocean) lies between the northern and southern sub-tropical convergences, where a strong water density gradient hinders nutrient recycling between deep layers and upper surface layers. The resulting low levels of new primary production make these zones the marine equivalent of deserts. Therefore, fisheries in this biome rely mainly on large pelagic fish, especially tunas, capable of migrating over the long distances that separate isolated food patches. In the eastern tropical Pacific, a major portion of the tuna purse-seine catch results from exploitation of a close association with pelagic dolphins, which suffered severe depletion in the 1970s due to incidental kills in the tuna purse seines (Gerrodette 2002). Between 1990 and 2000, 1.5 to 3.5 million Northeastern spotted dolphins (Stenella attenuata) were incidentally captured annually in tuna seine nets (Archer et al. 2002).
One exception to the general low productivity of the tradewinds biome is around islands and seamounts, where physical processes such as localized upwelling allow for localized enrichment of the surface layer. Above seamounts, these processes also lead to the retention of local production and the trapping of advected plankton, thus turning seamounts into oases characterized by endemism and, when pristine, high fish biomass.
Exploitation of the demersal resources of seamounts usually occurs in the form of intense trawling pulses, mainly by distant water fleets, which reduce biomass to extremely low levels, reduce diversity in the associated pelagic systems, and destroy biogenic bottom structures and their associated benthic diversity.
Similar exploitation occurs along ocean ridges, such as in the North Atlantic and the Central Indian Ocean, where poorly documented bottom trawl fisheries developed in the 1990s outside of any regulatory regimes.
Overall, the trade-winds biome contributed 15% of the world’s marine fisheries catch in 2001. Of this, 34% consisted of large pelagic fish and the rest were largely deep demersal species.
18.2.4 Westerlies Biome
In the westerlies biome (35.7% of the world’s oceans), seasonal differences in the depth of the mixed layer result from seasonality in surface irradiation and wind stress, inducing strong seasonality of biological processes, including a spring bloom of phytoplankton. The fisheries of this biome, mainly targeting tuna and other large pelagic species, are similar to those of the trade-winds biome.
The westerlies and trade-winds biomes are also inhabited by an enormous number of small mesopelagic fish that aggregate during the day at depths of 500–1,000 meters, forming a dense layer of fish and invertebrates, especially squid, and that migrate upward every night to feed on zooplankton at the surface layer (vertical migration). Their aggregate biomass, almost 1 billion tons (Gjøsaeter and Kawaguchi 1984), has often been described as a potential resource enabling further fisheries development. However, mesopelagic fish rarely occur in fishable concentrations, and their bodies tend to contain large amounts of wax esthers, which render their flesh unpalatable to humans.
Overall, the westerlies biome contributed 15% of the world’s marine fisheries catch in 2001. Of these, 9% were large pelagic fish with the rest consisting of small pelagic fish (40%), demersal fish (23%), and squid (11%). The marine environment in this biome is relatively unaffected by human use other than fishing. However, the large pelagic fish, such as tuna and shark, are strongly exploited.
18.2.5 Polar Biome
The polar biome covers only 15% of the world ocean and accounts for 15% of global marine fish landings. Its vertical density structure is determined by low-salinity waters from spring melting of ice. The bulk of annual primary production occurs in ice-free waters during a short intense summer burst. However, primary production under lighted ice occurs over longer periods, especially in Antarctica.
The Arctic fisheries along the north coast of Siberia, Alaska, and Canada (FAO Area 18) are poorly documented, and the few thousand tons of landings reported for this area by FAO are likely to be underestimates. The Arctic marine system is important for the well-being of indigenous people living in the area. For instance, marine mammals, such as whales and seals, are an important source of food and are of significant cultural value. However, high levels of persistent organic pollutants in their blubber pose a health concern. (See also Chapter 25 (Ecosystems and Human Well-Being: Volume 1: Current State and Trends: Marine Systems).) Climate change (Climate Change (collection)) has the potential to have a significant impact on the people of this area, since the ice forms a fundamental part of subsistence, shelter, travel, safety, and culture in the region. Oil and gas exploitation pose another set of issues for inhabitants of the Arctic (through social changes, for instance) and the ecosystem (through impacts on marine mammals, habitat damage or changes, oils spills and contamination).
The Antarctic krill, Euphausia superba, consumes the primary production from both open waters and under the ice and then serves as a food source for a vast number of predators, notably finfish, birds (including penguins), and marine mammals. As in the Arctic, the marine mammal populations of Antarctica were largely decimated before the middle of the twentieth Century.
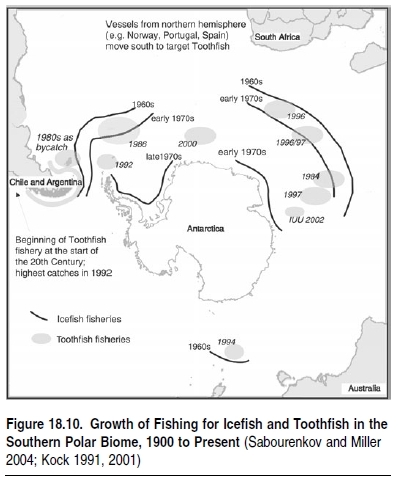
There is also a relatively small direct fishery for krill (about 150,000 tons per year) in Antarctica, which may expand if krill proves a suitable feed for salmon or other forms of farming. The development of fisheries in the southern polar biome demonstrates the fragility of fish stocks, marine mammals, and seabirds in terms of the impacts of exploitation by humans in just over 30 years. The distant water fleet of the former Soviet Union began exploiting this biome in the mid-1960s when ships began to deplete stocks of Marbled notothenia (Notothenia rossii), Mackerel icefish (Champsocephalus gunnari), and Gray notothenia (Lepidonotothen squamifrons) in different areas of the South Indian, South Atlantic, and South Pacific Oceans. (See Figure 18.10.) In all these areas, the same catch trends emerged: within a few years of opening fisheries, catches would peak and then rapidly decline to a small fraction of their original biomass. This operating mode of distant water fleets, including those of Russia, Chile, Argentina, France, and the United Kingdom, continued until the beginning of the 1990s.
The formation of the Commission for the Conservation of Antarctic Marine Living Resources in 1982 brought the first conservation measures for stocks of Marbled notothenia (in 1985). Other stocks remained unmanaged until the 1990s, when dramatic declines in stocks of Mackerel icefish at South Georgia and Kerguelen were recognized. Assessment, management, and control of the fisheries became much more stringent (Constable et al. 2000), and Mackerel icefish around South Georgia recovered sufficiently to allow limited commercial catches from the mid- 1990s onward (CCAMLR 2002). However, Kerguelen stocks have remained at a very low level (Duhamel and Claudet 2002).In the second half of the 1980s, the Soviet Union developed a longline fishery on Patagonian toothfish Dissostichus eleginoides (Kock 1991, 1992). The same declining catch trend for these long-lived, slow-growing species emerged for the stock around the Prince Edward Islands, which was reduced to very low levels within a few seasons. As a side effect, a large numbers of seabirds (such as albatrosses) became hooked on lines during the process of setting and hauling (Kock 2001). The situation became more aggravated when longline fishing was extended to virtually all grounds in the northern part of the southern polar biome from 1996/97 onward, and concern over the sustainability of stocks grew. Nevertheless, illegal, unregulated, and unreported fishing on the highly prized Patagonian toothfish increased dramatically from 1997 onward, and it is estimated that 80–90% of the current catch is taken illegally (CAMLR 2002).While it was possible to reduce IUU fishing around South Georgia to low levels, fishing pressure by IUU vessels remained high on other fishing grounds, notably in the Southern ocean, in FAO Area 58, despite considerable efforts by France and Australia to improve surveillance around the territories under their control. Commission members are working in closer cooperation with countries to assist with the apprehension of IUU vessels (as, for example, in the Viarsa incident; see www.intrafish.com). New fisheries are still being developed in the southern polar biome despite the lessons learned about the vulnerability of the local fish (including the Patagonian toothfish) to high levels of exploitation. Thus, New Zealand started an exploratory fishery on Antarctic toothfish (Dissostichus mawsoni) in the Ross Sea in the 1998/99 season (CCAMLR 2002) and has increased catches every year since.
18.2.6 Marine Biodiversity
18.2.6.1 Global Trends
This section provides a brief overview of marine biodiversity (Global marine biodiversity trends) in the context of fishing. [[Chapter 4 (Ecosystems and Human Well-Being: Volume 1: Current State and Trends: Marine Systems)]2] gives a broader view of marine biodiversity trends.
Assessing the biodiversity of the oceans has not been completed, both in general terms and with respect to specific system types, such as rocky grounds on continental slopes (Carlton et al. 1999). The factors influencing species distributions and patterns of species richness are only just emerging for widespread habitats such as soft sediments (MacPherson 2002; Gray 2002). Similarly, methods for measuring diversity and its patterns are evolving rapidly, so that in the future, if such methods are put into practice and applied in research and monitoring activities, our understanding of the condition and trends of marine biodiversity will improve significantly (Price 2002; MacPherson 2002; Warwick and Clarke 2001; Warwick and Turk 2002).
Information on commercially important or threatened species required for management purposes is quite limited. New non-fish species are frequently discovered and described in association with fisheries (Economics of fisheries) surveys or, more recently, environmental impact assessments. However, recent initiatives, such as the Census of Marine Life, are increasing the rate at which new knowledge on marine life is becoming available, although understanding of most taxa other than fish is very limited and reflects a failure to seek any systematic understanding of fisheries systems. For some groups important in fisheries catches, notably finfish and cephalopods, online databases that provide information on all species described so far do exist (see www.fishbase.org and www.cephbase.org). Also, a fair understanding of the factors influencing the distribution (depth, temperature, and so on) and population dynamics of major commercial species is available.
It is widely assumed that marine fish and invertebrates are somewhat less susceptible to extinction than most other marine as well as terrestrial and freshwater organisms. However, recent advances in methodology allowed studies that have questioned this assumption (Dulvey et al. 2003; Hutchings 2000). Although few marine species are known to have become globally extinct in the last century, there are numerous instances of extirpations of marine fish species—for example, the European sturgeon (Acipenser sturio) in the North Sea and the Green wrasse (Anampses viridis) in Mauritius.
Recent analyses suggest that marine extinctions may have been underestimated because of low detection abilities and a generally poor understanding of the conservation status of species that live in the marine realm (Dulvey et al. 2003; Carlton et al. 1999). Moreover, given that a major cause of declines or local extinctions in marine populations is overexploitation and that exploitation is rapidly increasing in scope and volume, there is a real likelihood that extirpations will increase (Dulvey et al. 2003). Other factors such as environmental degradation and climate change (Climate Change (collection)), alone or in combination with exploitation, can also play a role in local extinctions. As the first step toward global extinction, extirpations (local extinctions) cannot be dismissed as unimportant or irrelevant to a species’ status and have significant impacts on the provision of ecosystem services. (See Chapters 4 (Ecosystems and Human Well-Being: Volume 1: Current State and Trends: Marine Systems) and 11 (Ecosystems and Human Well-Being: Volume 1: Current State and Trends: Marine Systems).)
The now rapidly fading notion that marine fish and invertebrates are inherently more resilient to impacts on their populations than other wildlife was based on a number of unfounded assumptions about their biology, particularly in the case of species that release large numbers of eggs into the open water. The key assumption was that high fecundity combined with a seemingly high dispersal capacity of the eggs or larvae, high recruitment variability, and wide-ranging distributions minimizes the risk of extirpations or extinctions even under heavy fishery exploitation. However, scientific support for this assumption is lacking or poor. Indeed, there is now an emerging consensus that marine fish are no more resilient to extirpations or extinctions than any other wildlife species of similar size (Roberts and Hawkins 1999; Hutchings 2000; Sadovy 2001).
18.2.6.2 Ecosystem and Habitat Diversity
The number of species present and their relative abundance is an important aspect of biodiversity and is threatened in marine systems. Overfishing and destructive fishing methods have an impact on marine ecosystems by changing community structure and altering trophic and other interactions between ecosystem components and by directly modifying habitats, notably when trawlers erode biogenic bottom structures (Pandolfi et al. 2003). By removing important components of the ecosystem, such as algal feeding fish in coral reef (Coral reefs (collection)) (Coral reefs (collection)) systems, overfishing results in altered ecological states that may be impossible to restore to former conditions. (See [[Chapter 19 (Ecosystems and Human Well-Being: Volume 1: Current State and Trends: Marine Systems)]5].)
A number of generalities can be drawn from the literature on biodiversity. One is that biological production declines with increasing ‘‘trophic level’’ (the number of feeding levels that organisms are removed from phytoplankton and other primary producers; see Chapter 8 (Ecosystems and Human Well-Being: Volume 1: Current State and Trends: Marine Systems) for an explanation of the trophic level concept). In fisheries, most catches occur at around trophic level 3, which consists of small fish (such as sardines and herrings) feeding on herbivorous zooplankton (zooplanktivorous fish), and around trophic level 4, which consists of fish that prey on the zooplanktivorous fish (such as cods and tunas). Many fish, however, have intermediate trophic levels, as they tend to feed on a wide range of food items, often feeding on zooplankton as juveniles and other fish when adults. (See www.fishbase.org for diet composition data and trophic level estimates on thousands of fish species and the corresponding references.)
Biomass energy is transferred up the food web, with transfer efficiencies between trophic levels ranging, in marine ecosystems, from about 5% to 20%, with 10% a widely accepted mean. Thus the productivity of the large, high trophic–level fish that are traditionally targeted will always be lower than that of lower trophic– level fish. This has led to suggestions that fisheries yields should be increased by deliberately ‘‘fishing down the food chain’’ (Sprague and Arnold 1972)—that is, by exploiting species located at lower trophic levels more intensively. But this is already occurring throughout the world’s oceans as a result of the decline in catches of the large, slow-growing high trophic–level fish, which are gradually being replaced, in global landings, by smaller, shorter-lived fish, at lower trophic levels (Pauly et al. 1998). Unfortunately, fishing down marine food webs does not necessarily lead to increased catches (see earlier Figures 18.3 and 18.9). Indeed, globally both the landings and their mean trophic levels are currently falling under the pressure of fisheries (Pauly et al. 1998; Watson and Pauly 2001); what seems to be increasing worldwide is the abundance of jellyfish, which are increasingly exploited throughout the world and exported to East Asia.
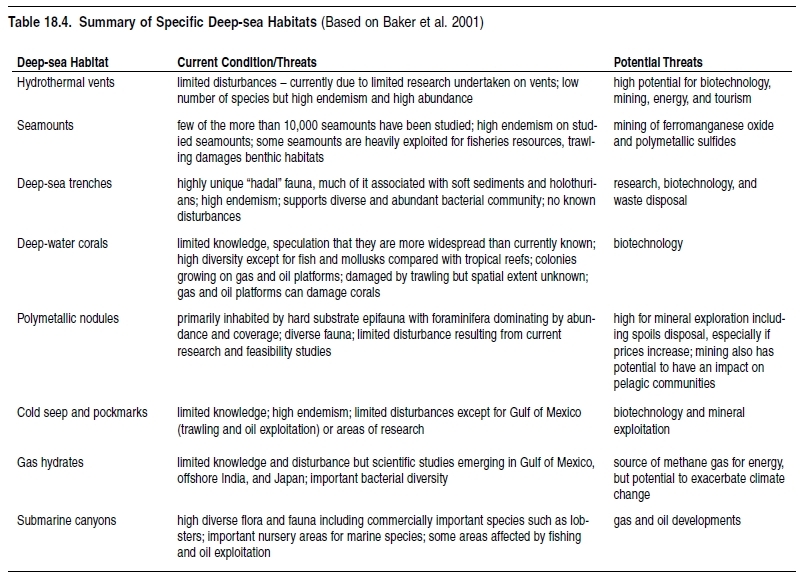
The deep ocean bottom contains some of the least explored areas of the world, with only 0.0001% of the deep seabed subject to biological investigations thus far (WWF/IUCN, 2001; Gray 2002). Nevertheless, studies have revealed a wealth of diverse habitats in the deep sea, which include seamounts, cold-water coral reefs, hydrothermal vents, deep-sea trenches, submarine canyons, cold seeps and pockmarks, and gas hydrates and polymetallic nodules. Of those, seamount ecosystems and cold-water coral reef communities are particularly threatened by high-impact fishing methods, such as bottom trawling (Thiel and Koslow 2001; Freiwald et al. 2004).
Scientific exploration of seamounts is minimal, with only approximately 300 of them sampled biologically, out of what is believed to be tens of thousands worldwide (ICES, 2003; see also seamounts.sdsc.edu). As mentioned previously, seamounts increase the biological productivity of waters surrounding them.The tops and upper flanks of seamounts also tend to be biological hotspots, with potentially high species diversity and endemism. Marine mammals, sharks, tuna, and cephalopods all congregate over seamounts to feed, and even seabirds have been shown to be more abundant. Suspension feeders, such as corals, dominate seamount benthic fauna. Seamounts may also act as ‘‘stepping stones’’ for transoceanic species dispersal (WWF/IUCN, 2001).
Our knowledge of cold-water coral diversity is also limited, and new reefs are still being discovered. For example, the largest known cold-water reef—35 kilometers long and 3 kilometers wide—was discovered off the Norwegian coast in June 2002 (Freiwald et al. 2004). There are few quantitative studies of fauna associated with cold-water corals, but it is known that they provide habitat for high diversity of associated species. More than 800 species have been recorded in the Lophelia pertusa reefs in the northeast Atlantic, and 3,000 species of fish and mollusks have been identified on deepwater reefs in the Indo-West Pacific region (WWF/IUCN, 2001).
The biggest threat to deep-sea coral reef (Coral reefs (collection)) (Coral reefs (collection))s (Coral reefs (collection)) comes from trawling activities. WWF (2002) suggest that 30–50% of the deep-water corals along the Norwegian coast have already been lost due to bottom trawling, marine pollution, and oil and gas exploration. Inconsistent and opportunistic sampling in deep and isolated areas, where cold waters and deep-sea corals are located, hampers efforts to study these habitats, and it is likely that global assessments will underestimate the biodiversity of these areas. More is known about local habitats and local extinctions in warm waters, such as the loss of the sawfish (Pristis pectinata) in Mauritania (UNEP 2002b) and the Chinese bahaba (Bahaba taipingensis) in Hong Kong (Sadovy and Cheung 2003).
18.2.6.3 Species Diversity
The lowered biomass and fragmented habitats resulting from overexploitation of marine resources is likely to lead to numerous extinctions, especially among large, long-lived, late-maturing species (Sadovy and Cheung 2003; Sadovy et al. 2003a; Denney et al. 2002).
Fishing is thus one of the major direct anthropogenic forces that has an impact on the structure, function, and biodiversity of the oceans today. Climate change (Climate Change (collection)) will also have impacts on biodiversity through changes in marine species distributions and abundances. In the coastal biome, other factors, including water quality, pollution, river and estuarine inputs, have large impacts on coastal and marine systems. (See [[Chapter 19 (Ecosystems and Human Well-Being: Volume 1: Current State and Trends: Marine Systems)]6].) Historical overfishing and other disturbances have caused dramatic decreases in the abundance of large predatory species, resulting in structural and functional changes in coastal and marine ecosystems and the collapse of many marine ecosystems (Jackson et al. 2001).
One well-documented example is that of the historic fishing grounds ranging from New England to Newfoundland and Labrador, which once supported immense cod fisheries but which have now been almost completely replaced by fisheries targeting invertebrates, the former prey of these fish (providing a classic example of fishing down marine food webs). The system that once supported cod has almost completely disappeared, fueling fears that this species will not rebuild its local populations, even though fishing pressure has been much reduced (Hutchings and Ferguson 2000; Hutchings 2004; Lilly et al. 2000). However, some collapsed stocks have been able to recover once fishing pressure is removed: the North Sea herring fishery collapsed due to overharvest in the late 1970s but recovered after a four-year closure (Bjørndal 1988). On a much smaller scale, but nevertheless widespread throughout the tropics, coral reef areas have been degraded by a combination of overfishing, pollution, and climate variability. (See [[Chapter 19 (Ecosystems and Human Well-Being: Volume 1: Current State and Trends: Marine Systems)]7].)
18.2.6.4 Genetic Diversity
An important component of biodiversity is genetic diversity (FSBI 2004). Even for those marine groups that are taxonomically well documented, relatively little is known about the subdivision of species into populations with distinct genetic (and sometimes morphological) features, which are of evolutionary importance and of potential human use. Lack of knowledge about appropriate conservation units can lead to inadvertent overexploitation of distinct populations and to their extirpations (Taylor 1997; Taylor and Dizon 1999); where recovery is possible, it may take decades or centuries, as in the case of some populations of large species of whale (Clapham et al. 1999). In some cases, genetic diversity may be irretrievably lost due to a ‘‘bottleneck’’ effect caused by overexploitation, as with the northern elephant seal (Mirounga angustirostris) population, which was nearly exterminated by early commercial sealing (Bonnell and Selander 1974; Stewart et al. 1994).
18.3 Drivers of Change in Marine Fisheries Systems
There are two direct drivers and several indirect drivers of changes in marine ecosystems. The climate, due to its natural variability and increasingly because of greenhouse gas emissions, drives a number of changes affecting marine ecosystems, while government policy primarily drives change through the effect on investment in fisheries, with direct drivers such as overfishing resulting from government subsidies. Economic factors, including an increase in demand reflected in an increase in price and food preferences, also affect fisheries, with population growth exacerbating most of these.
18.3.1 Climate Change
Climate change (Climate Change (collection)) is a direct driver in marine systems (McLean et al. 2001) and its potential impacts are described later, in the section on choices and trade-offs. Changing wind patterns and sea temperatures have an impact on various oceanographic processes, including upwellings (for example, Benguela) and surface currents (as in the Gulf Stream) (McLean and Tsyban 2001). (See also Chapters 12 (Ecosystems and Human Well-Being: Volume 1: Current State and Trends: Marine Systems) and 13 (Ecosystems and Human Well-Being: Volume 1: Current State and Trends: Marine Systems).) These currents may slow down, shift spatially or disappear altogether, resulting in changes in population abundance and distribution for many marine species.
There may be local extirpations, but global extinctions in the oceans are unlikely to result from climate change alone. Recent results from monitoring sea temperatures in the North Atlantic suggest that the Gulf Stream may be slowing down and affecting abundance and seasonality of plankton that are food for larval fish (Richardson and Schoeman 2004). Declining larval fish populations and ultimately lower adult stocks of fish will affect the ability of overexploited stocks to recover (Beaugrand et al. 2003).
Climate-induced changes in their physical characteristics (such as currents and circulation patterns) and their chemical characteristics (such as nutrient availability) will affect marine ecosystems directly. These impacts include sea surface temperature–induced shifts in the spatial distribution of some species and compositional changes in biodiversity, particularly at high latitudes. A poleward shift of marine production due mainly to a longer growing season at high latitudes is anticipated. While a complete shutdown of the North Atlantic circulation is unlikely, it cannot be ruled out, even in the foreseeable future (IPCC 2003).
A poleward shift of marine production due mainly to a longer growing season at high latitudes is anticipated (IPCC 2003). Recent findings show that warming in the Northern Hemisphere will cause a northern shift of distribution limits for various species through improved growth and fecundity in the north and lower growth or even extinctions in the south of this range. Such shifts may seriously affect fishing activities in the North Sea (Portner et al. 2001) and other productive areas of the world’s oceans. However, current knowledge of the impacts of climate change in marine ecosystems is still poor, and literature on the subject is scarce. Current scenarios of global climate change include projections of increased upwelling and consequent cooling in temperate and sub-tropical upwelling zones. Such cooling could disrupt trophic relationships and favor less complex community structures in these areas (Aronson and Blake 2001; Barret 2003). Marine export production may be reduced (estimated at -6%), although regional changes may be either negative or positive (from -15% zonal average in the tropics to +10% in the southern polar biome) (Bopp et al. 2001).
18.3.2 Subsidies
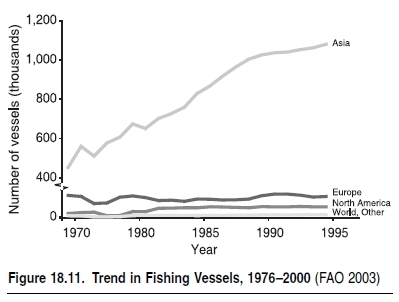
There are two forms of subsidies: direct financial support in industrial countries (price supports, for instance) and indirect support, notably in the form of open access policies that allow resource rents to be spent on excess capacity. The latter happens when the surplus of funds from a fishery after all costs have been paid are spent on purchasing additional capacity.Financial subsidies are considered to be one of the most significant drivers of overfishing and thus indirect drivers of change in marine ecosystems. In most cases, government subsidies have resulted in an initial increase of overall effort (number of fishers and size of fleet), which translates into increased fishing pressure and overexploitation of a number of species. While it appears that the number of fishing vessels (see Figure 18.11) and fishers stabilized in the late 1990s, other subsidies, e.g. cheap fuel subsidies, can keep fleets operating even when fish are scarce. Without such subsidies, many of these fisheries would cease to be economically viable (Munro and Sumaila 2002).
Subsidies also play a role in fisheries expansion. Globally, the provision of subsidies to the fisheries industry has been variously quantified at $20 billion to over $50 billion annually, the latter roughly equivalent to the landed value of the catch (Christy 1997). More conservative estimates are provided by Milazzo (1998) and by an OECD (2000) study, recently reanalyzed and scaled to the North Atlantic by Munro and Sumaila (2002). The latter suggested an annual subsidy of $2.5 billion for a part of the world ocean that contributes about one sixth of the world catch.
The subsidies given to fisheries vary between countries and range from unemployment benefits in Canada to tax exemption in the United States and payment of fees to gain access to foreign fishing grounds by the European Union (Kaczynski and Fluharty 2002). For instance, in 1997 Canada provided over $198 million in unemployment benefits to its fishing sector; the United States gave $66 million in tax exemptions, and the European Union provided subsidies of $155 million to obtain access to other countries fishing grounds. Each of these have the effect of either reducing the cost of fishing or increasing the net revenues fishers obtain, and hence they lead to more fishing than would have been the case without the subsidies.
Over half the subsidies in the North Atlantic have negative effects on fleet development (Munro and Sumaila 2002). This, perhaps surprisingly, includes decommissioning subsidies, which have been shown under most circumstances to have the effect of helping to modernize fleets, thereby bringing about an increase in their catching powers.
18.3.3 Demand and Fish Prices
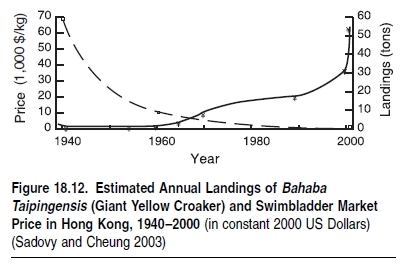
Overfishing drives ecosystem change, including changes in biodiversity, as described earlier. The growing demand and correspondingly increase in prices has contributed to overfishing.Marine products are in demand as a luxury food as well as for subsistence in many coastal communities, and as feed for aquaculture and livestock. It is the relatively high prices for these products combined with subsidies (plus the use of coastal systems as disposal sites for their waste products) that makes aquaculture a feasible industry.It has been reported that bluefin tuna (Thunnus thynnus) have sold on the Tokyo market for as much as 20 million yen for a single fish (Japan Times 2001). Other fishery products such as eel larvae (Anguilla spp) and large prawns are also extremely high priced commodities. Such very high prices generate extreme pressures for overexploitation that are sometimes nearly impossible to counter through local management measures. As such items become increasingly scarce, they increasingly assume the status of luxury foods. The result is that increasing scarcity, rather than causing a relaxation of pressure on the remaining remnants of the resource populations, may act to increase the incentives to harvest the remaining individuals. For example, the Chinese bahaba (Bahaba taipingensis) is highly sought after for its swimbladders used in traditional medicine. Consequently, this fish—which fetches $20,000–64,000 per kilogram (see Figure 18.12)—has been exploited to critically low levels (Sadovy Cheung 2003).
18.3.4 Shifting Food Preferences and Consumption
It could be argued that human population growth and the resulting need for inexpensive food have been driving fisheries expansion. However, human population growth did not drive excess fleet capacity from Northern Europe and Japan into the southern oceans. Human population growth did not stimulate people in countries not accustomed to eating fish to shift toward a heavy consumption of seafood, as seen in China where income growth and urbanization has fueled fish consumption (Delgado et al. 2003). In industrial countries, such as the United States, fish is no longer a cheap source of protein compared with other sources. The price of fish has increased in real terms while the price of red meat has lost half its value over the last 20 years (Delgado et al. 1999).
Factors driving overfishing other than human population growth are also at work. One of these is increase in incomes and therefore fish consumption in various countries that previously did not appear in international markets, such as China (Ahmed et al. 1999). Another factor is the consumption of fish promoted as part of a ‘‘healthy’’ diet and changing food preferences in many industrial countries.
18.3.5 Technological Change
Historically, the global expansion of fisheries has been driven by successive waves of technological innovation, much of it developed for naval warfare following the Industrial Revolution, two World Wars, and the cold war. These innovations included the invention of steam and diesel engines, the onboard manufacturing of ice, and blast freezing, all of which expanded the range of industrial fishing vessels. This expansion was followed by the incorporation of an enormous array of electronic devices facilitating fish detection, including radar and acoustic fish finders on fishing vessels, culminating in the introduction of GPS technology and detailed seabed mapping at the end of the cold war. These technologies, while improving the safety of people working at sea, also allowed fishers to aim for specific places with high fish abundances, places that once were protected by the depths and vastness of the oceans.
18.3.6 Illegal Fishing
The profits of fisheries that choose to operate outside of national and international laws and conventions can be very high. In some areas there is a lack of surveillance, enforcement, and monitoring due to high operational costs. In other areas corruption and cheating are tolerated due to the economic conditions or social obligations within a country. Managers recognize illegal, unreported, and unregulated fishing as a global problem, and recent initiatives, such as FAO’s International Plan of Action, will help formulate strategies to deal with the problem. Regional fishery management organizations, such as the International Commission for the Conservation of Atlantic Tunas, are dealing with nonmembers and members who do not comply with management measures through the use of economic sanctions. ‘‘Name and shame’’ strategies are being used by NGOs to force companies and governments to comply with international management measures (for example, the Coalition of Legal Toothfish Operators, see www.colto.org/ vessels.htm).
Large-scale cheating in some fisheries that were supposedly regulated internationally has led to extreme depletion of some living marine resources. An especially egregious example is the misreporting of Soviet whale catches to the International Whaling Commission (Brownell and Yablokov 2002). Between 1947 and 1972, some 90,000 more whales were taken in the Southern Hemisphere than was reported, including more than 3,000 endangered southern right whales that were supposedly fully protected at the time. Similar violations occurred in the North Pacific and Indian Oceans, and it was only with the introduction of international on-board observers that these practices ceased. It is now widely agreed that independent surveillance is an essential part of any fishery management and enforcement plan.
18.3.7 Globalization (Environment and Globalization: The Five Propositions)
Fish represent the fastest-growing food commodity entering international trade (Preston 1997). Accordingly, fish and fish products are an extremely valuable source of foreign exchange to many countries, in some cases providing as much as half of their total available foreign exchange income. For example, in Guinea- Bissau fishing agreements with the EU finance more than 45% of the government’s annual fisheries operations budget, though this 492 Ecosystems and Human Well-being: Current State and Trends country only receives a very small fraction of the value ( 10%) of the fish taken by European fleets (Kaczynski and Fluharty 2002).
Stocks of bluefin and other large tuna species around the world are being strained by fishing pressure driven by the extremely high prices such fish fetch in the Japanese luxury fish markets. Traditional local fish foods are, in many cases, no longer available to local consumers due to their inability to match the prices available by shipping the products elsewhere. An example is Senegal, where exports have disrupted local supplies of fish (UNEP 2002a). Consequently, highly nutritious fish foods produced in poorer regions of the world are increasingly being eaten by more economically advantaged populations in distant areas of North America, Europe, and East Asia. Of particular concern is the East and Southeast Asian market for shark fins that is threatening many shark species around the world, which are already under pressure from being a significant part of the bycatch of many pelagic fisheries.
One benefit of globalization is the improved quality of fish, because most importing countries demand that exporting facilities meet Hazard Analysis and Critical Control Point standards, which require exporting countries to follow safe food processing and handling standards. The associated benefits have been mainly to industrial countries, however. In developing countries, benefits have been limited to companies that can afford the required investment (Atta-Mills et al. 2004) or to the few local fishers able to participate in ‘‘boutique’’ fisheries for live fish, seahorses, and aquarium fish, which are low volume but a high-price export product (Erdmann and Pet-Soede 1996; Tomey 1997; Sadovy and Vincent 2002; Alder and Watson in press).
Export fisheries have also influenced the aquaculture industry, especially for salmon and shrimp, which are bred to meet the demand from industrial countries for luxury high-value seafood. For example, salmon (much of it farmed) was the leading fish export commodity of the EU in 1998 (Smith and Taal 2001).
Countries such as Thailand that are the leading producers of shrimp (much of it from aquaculture) are often the leading exporters. Increasing exports have contributed to the expansion of fishing fleets (facilitated by subsidies) leading to overcapacity and overexploitation as seen in the development of the pollock industry in the 1980s in Alaska (St Clair 1997). Depending on the fishery, this can lead to habitat destruction through trawling and biodiversity loss through, for example, turtles caught in shrimp trawls, albatross and sharks caught by longlines, and other bycatch in various fisheries (Hall et al. 2000).
Globalization clearly has the benefit of supplying foreign exchange to developing countries and potentially decreasing national debt. But this benefit has been at the cost of domestic supplies of fish resources, resulting in increasing domestic prices; in India, for example, the cost of fish has increased faster than the cost-of-living index and other meats (Kurien 1998) and has decreased food security (Marine biodiversity and food security).
18.3.8 Other Drivers
Habitat changes in coastal systems are a major driver of fisheries declines. (See [[Chapter 19 (Ecosystems and Human Well-Being: Volume 1: Current State and Trends: Marine Systems)]8].) Other factors of lesser apparent importance are invasive species, pollution, and disease. Human impacts, especially exploitation, are increasing. Moreover, persistent and widespread misconceptions about the ability of marine fish populations to withstand and recover from fishing continue to undermine initiatives to address the root causes of these problems (Roberts and Hawkins 1999; Hutchings 2004). Habitat loss or damage is caused by a range of fishing practices (from bottom trawling to the use of dynamite), by pollution, or possibly by global warming, as in the case of extensive bleaching of coral reef (Coral reefs (collection)) (Coral reefs (collection))s (Coral reefs (collection)). Even well-intended attempts to remediate declines in fisheries through stocking can be problematic as hatchery operations have an impact on the genetic structure of wild stocks.
Two additional processes have effects similar to subsidies. One is the rapid increase in the demand for fish, reflected in increased prices of fish products, which in the last 50 years have increased three to four times faster than the consumer price index (Delagado and Courbois 1999). The other is the low price of fuel, which keeps numerous, otherwise bankrupt fisheries afloat in many countries. Moreover, due to the decline in stock abundance, the catch and edible protein per amount of fuel burned has decreased over time (Tydemers 2004). Indeed, fisheries are probably the only sector of the economy that has decreasing fuel efficiency (compared with, say, trucking, aviation, or manufacturing). Obviously, this growing dependence of the fishing industry on cheap fuel makes it highly vulnerable to fuel price increases, as well as to implementation of the Kyoto protocol or similar agreements that would tax industries for their energy intensity (Pauly et al. 2003).
18.4 Choices, Trade-offs, and Synergies within the System
Marine systems are still considered a new frontier for development by some people (McNutt 2002), and therefore a number of choices and trade-offs over fisheries will need to be made in the future. History has shown that once humans exhaust resources on land they look to the sea for alternatives. In repeating history, coastal environments are becoming degraded (for loss of coral reefs, see [[Chapter 19 (Ecosystems and Human Well-Being: Volume 1: Current State and Trends: Marine Systems)]9]) and biodiversity is declining, beginning with the loss of large predators at high trophic levels (Pauly et al. 1998; Myers and Worm 2003). Now areas deeper and further offshore are increasingly exploited for fisheries and other resources such as oil and gas.
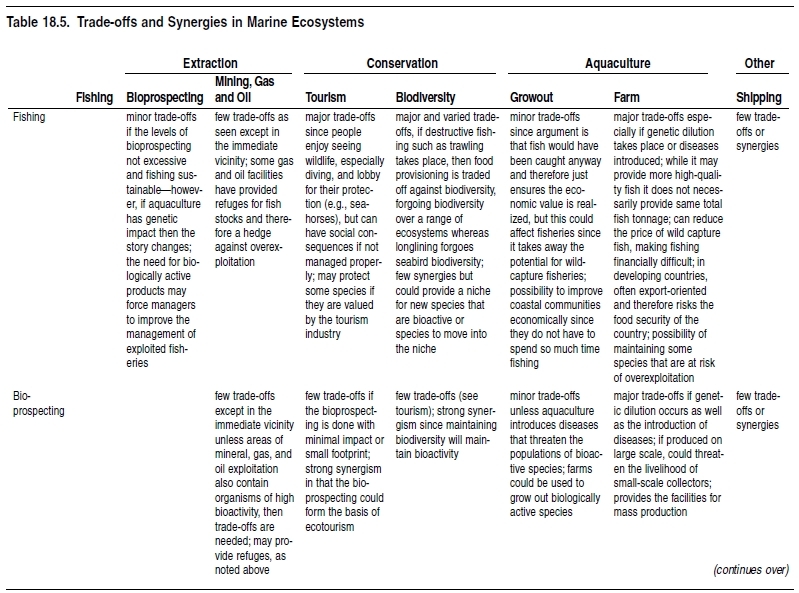
Marine fish resources often have value and benefits beyond that of food security (Marine biodiversity and food security). Some species are of considerable cultural importance (salmon are an important part of aboriginal culture in the Northeast Pacific, for instance), while others generate substantial income from tourism (especially dive tourism) and recreation (Rudd and Tupper 2002). Yet others may be important keystone species within their community, with a loss even at local levels cascading throughout the ecosystem. (See Chapter 11 (Ecosystems and Human Well-Being: Volume 1: Current State and Trends: Marine Systems).) These trade-offs need to be considered when allocating resource access. Nevertheless, there are some uses of marine systems that have minimal impacts and that can be developed in tandem with other uses such as tourism, well-managed recreational fisheries, and bioprospecting. (See Table 18.5.)
18.4.1 Environmental Impacts of Capture Fishing versus Other Uses
Contrary to the coastal systems, where many uses are mutually incompatible, few other economic activities in the marine realm directly preclude fishing. In fact, the major problem for fishers is other fishers. Thus, for example, by modifying habitats, trawlers affect the yield of other fishers who do not use such destructive gear.Three different classes of multiple uses and synergies can be identified:
- relationships between fisheries and other sectors, such as aquaculture and coastal development;
- relationships between fisheries and top predators or charismatic fauna (marine mammals, seabirds, turtles); and
- competition within the fisheries sector.
Generally, fisheries do not appear to be affected to a large extent by other extractive activities, such as oil or seabed mining, at least relative to the wide impact of the fisheries themselves.
The issue of competition with humans does not arise with marine turtles, which along with marine mammals and seabirds are key indicator species for problems and changes in the marine environment. (See [[Chapter 19 (Ecosystems and Human Well-Being: Volume 1: Current State and Trends: Marine Systems)]10] for more on marine wildlife.)
It has been proposed that marine mammals directly or indirectly compete with fisheries (commercial and artisanal) for resources targeted by fisheries. This perceived competition has been used to justify annual sustainable harvests of marine mammals during the last decade (Lavigne 2002) and also to justify the resumption of whaling in many international fora (Holt 2004). Though competition may occur at small local scales, this issue warrants much further investigation. A recent analysis of global trophic overlap between marine mammals and fisheries indicated that there is limited competition in the Northern Hemisphere on a large scale, while competition between the two is low in most other areas of the world (Kaschner 2004; Kaschner and Pauly 2004). Moreover, the analysis suggested that, overall, fisheries are more likely to adversely affect marine mammal species, particularly those with restricted ranges, than vice versa (Kaschner 2004).
Examples of marine mammals adversely affecting humans do exist. However, such impacts are far less severe and mostly fisheries- related, such as when killer whales take fish from the catch of longline fisheries in Alaska. Reducing the competition between higher vertebrates and fisheries is likely to involve both technological changes in the way fishing gears are deployed and the creation of suitably large marine reserves (as described later).
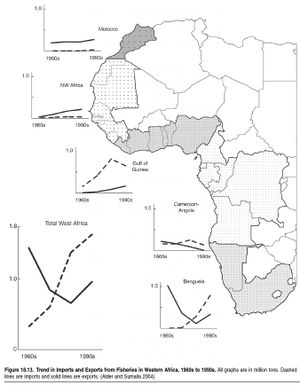
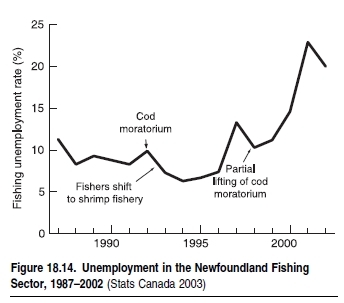
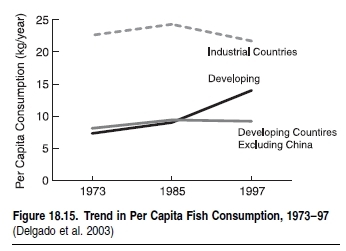
The third group of interactions occurs between fishers and fleets. Essentially, these interactions are shaped by the fact that each fisher looks for exclusive access to the resource. In fact, the technological improvements that characterize modern fisheries, and that enable access to resources deeper and further offshore, are a response to competition between fishers. This competition, which drives the technological development of fisheries, has over time eliminated the refuges, such as depth and distance offshore, that naturally protected fisheries resources (Pauly et al. 2002).The case study in Box 18.1 documents an example of how European Union subsidies for technological development and fleet improvements gave Mediterranean trawlers access to fish populations in previously out-of-reach areas of the deep sea. A significant proportion of world fish stocks and catches is overexploited or depleted (Watson and Pauly 2001; FAO 2002) and the marine habitats that many of the world’s fish stocks rely on at some stage of their life cycle are being degraded. (See [[Chapter 19 (Ecosystems and Human Well-Being: Volume 1: Current State and Trends: Marine Systems)]11].) The combination of overfishing and degradation or conversion of habitats, which contribute to the loss of biodiversity and food provisioning, occurs almost everywhere. In developing countries this is aggravated by export-driven fisheries that overexploit their resource base and that divert food away from the domestic market. (See Figure 18.13.) As a result, the fishing sector has declined as a source of employment in many industrial countries. 18.4.1.1 Food and ProteinOverfishing affects human well-being through declining food availability in the long term, since fewer fish are available for consumption and the price of fish increases (Alder and Sumaila 2004). Due to declines in coastal habitats, fishers are forced to go further offshore and for longer periods of time, resulting in reduced food security (Marine biodiversity and food security) (Alder and Christanty 1998). In Canada, the collapse of the cod fishery resulted in severe unemployment (see Figure 18.14), compounded by restrictions on subsistence fishing (Neis et al. 2000).While fish is a healthy, luxury food in high demand by the industrial world, it is still a significant and cheap source of protein for many countries in the developing world. However, per capita consumption of fish in the latter is much lower than in the industrial world. (See Figure 18.15.) Therefore declines in the availability of cheap fish protein either through overfishing, habitat changes, or shifting trade practices contribute to reduced food security in countries such as Ghana, Senegal, and Chile (Atta- Mills et al. 2004; Alder and Sumaila 2004).
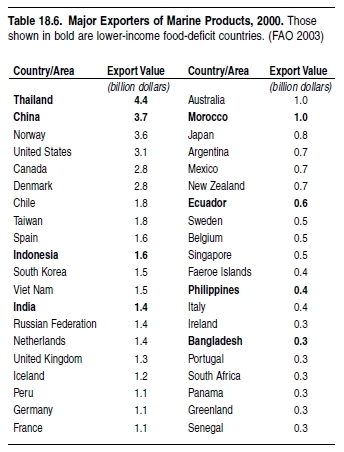
The developing world produces just over 50% of the value of fish that is traded globally, and much of the fish caught in the developing world is exported to industrial countries (FAO 2002). Fishing to meet export demands should, theoretically, provide funds to allow the import of cheaper fish and protein products, reduce the government debt, and supply cheap protein to the local population. However, the benefits to developing countries from trade in fish will not be realized if the funds generated are not reinvested in the economy. That this is not always the case may contribute to the fact that some of the major fish-exporting countries are also the least developed. (See Table 18.6.)
The industrial world, in particular the United States, the EU, and Japan, have been able to buffer against declines in fish availability and increases in prices because they have been able to purchase or otherwise get access to high-quality fish. Indeed, the per capita consumption of fish by industrial countries was 21.7 kilograms in 1997, compared with 9.2 kilograms in the developing world (excluding China, although if China is included, the per capita consumption for the developing world rises to 14 kilograms due to China’s massive consumption of locally produced farmed freshwater fish) (Delgado et al 2003).
18.4.1.2 Livelihoods
Fisheries and fish products provide direct employment to nearly 27 million people (FAO 2002). Globally, the bulk of people employed in fisheries are poor, and many are without alternative sources of work and sustenance. In addition, fish and fishing are enormously important to the cultural life of many coastal communities, and often define the ‘‘quality of life’’ of people with a cultural tradition of harvesting the sea (Johannes 1981).
|
BOX 18.1 Subsidy-driven Removal of a Natural Reserve The Mediterranean Sea has a long history of fisheries exploitation with a variety of gears. Yet the landings of many species, including highly valued demersals, have increased in the last decades (Anonymous 2002; Fiorentini et al. 1997). A large part of the landings consist of the juvenile of demersal species (Stergiou et al. 1997; Lleonart 1999; Lloret et al. 2001), such as hake, Merluccius merluccius—one of the six most important species, in terms of both landings and landed value, in the Mediterranean Sea (Fiorentini et al. 1997). Hake landings rose from about 5,000 tons in the late 1940s in the eastern and western Mediterranean to about 15,000 and 40,000 tons in the mid-1990s in two areas of the Mediterranean (Fiorentini et al. 1997). The increase in landings is not only the result of increasing fishing mortality (that is, increasing effort and technological developments), but also due to recent development of new fisheries and expansion of fishing grounds (mainly by fishing in deep waters). Increasing landings are also related to the increase in phytoplankton production due to eutrophication of Mediterranean waters (e.g., Caddy et al. 1995; Fiorentini et al. 1997; Tserpes and Peristeraki 2002). For some demersal species, increasing landings might have been mitigated by the fact that a large part of the spawning population was not available to fishing gears because of: • life history—with the onset of maturation, many demersal fish species (e.g., M. merluccius (Abello et al. 1997), Pagrus pagrus (Labropoulou et al. 1999), and Pagellus erythrinus (Somarakis and Machias 2002)) migrate to deep bottom areas that were not accessible to trawlers—and • the geomorphology of the Medi terranean Sea deep areas provides natural refuges (low-take zones), allowing mature individuals to contribute to recruitment and stock size maintenance (Caddy 1990, 1993, 1998; Abella et al. 1997). In the last several years, however, the modernization of trawlers and small-scale vessels, driven mainly by EU subsidies, allowed fishing to expand in deeper waters and to operate during harsh weather conditions. As a result, deepwater fisheries, practiced for instance with bottom or vertical longlines, were developed, heavily exploiting the large mature hake as well as of other species (e.g., Pagellus bogaraveo). Given the absence of appropriate management procedures (Lleonert 1999; Stergiou et al. 1997), this will lead to recruitment overfishing and the fisheries will eventually collapse, adding another data point to the records on the negative effect of subsidies. |
18.4.1.3 Habitats
Some coastal habitats have been converted to other uses, such as mangroves (Mangrove ecology) for coastal aquaculture ponds or cage culture of highvalued species such as penaeid shrimp, salmon, or tuna. This conversion can affect wild-capture fisheries, which use these coastal habitats for part of their life cycle. It also has sometimes caused displacement of fishers, loss of revenues, and social unrest. Coastal residents often no longer have access to cheap protein or sources of income. (See Figure 18.16) In addition growing juvenile fish taken from wild populations, in the case of tuna, in pens or cages can also be a significant source of pollution for the area in which they are located.
Area closures and the halt of destructive fishing have improved fisheries, especially in coral reef (Coral reefs (collection)) (Coral reefs (collection))s (Coral reefs (collection)) (Roberts et al. 2002). Overall, however, overfishing and habitat destruction continue throughout the world (Jackson et al. 2001; Jennings et al. 2001).
Ultimately, overfishing has a significant impact on most marine systems. Habitat degradation due to pollution, infrastructure development, and so on contributes to the further degradation of the ecosystem and declining human well-being (mercury and PCBs in Baltic Sea fish, for example) or impedes recovery of the marine or coastal system (as when the conversion of mangroves (Mangrove ecology) affects important nursery habitat for some species of fish). Considering the serious nature of many non-fisheries impacts, attention should be given to addressing these impacts and within the context of integrated coastal management. (See Chapter 15 of the MA Policy Responses volume).
Offshore areas, especially the deep sea, vents, and seamounts, are at risk of being exploited beyond recovery. It is difficult to say how much direct impact the crossing of such thresholds will have on human well-being. Nevertheless, it will ultimately affect ecosystem services. The lack of good information on these systems, past experiences regarding the impact of fishing, and lack of good international management frameworks add to the uncertainty of the impacts on these systems.Climate change, El Nino/Southern Oscillation, and hydrological conditions will have a significant impact on some marine habitats, especially coastal areas ([[Chapter 19 (Ecosystems and Human Well-Being: Volume 1: Current State and Trends: Marine Systems)]12]) and in polar marine and coastal systems ([[Chapter 25 (Ecosystems and Human Well-Being: Volume 1: Current State and Trends: Marine Systems)]2]) (Chavez et al. 1999). Climate change will affect marine systems but may not have as severe an impact on open-ocean and deep-water systems (McLean et al. 2001).18.4.1.4 National Economies and Foreign Exchange
Many areas where overfishing is a concern are also low-income, food-deficit countries. For example, the exclusive economic zones of Mauritania, Senegal, Gambia, Guinea Bissau, and Sierra Leone in West Africa all accommodate large distant water fleets, which catch significant quantities of fish. (See Figure 18.17 in Appendix A.) Much of it is exported or shipped directly to Europe, while compensation for access is often low compared with the value of the product landed. These countries do not necessarily benefit through increased fish supplies or increased government revenue when foreign distant water fleets access their waters. In some countries, such as Coˆte d’Ivoire, the landings of distant water fleets can lower the price of fish, which affects local small-scale fishers.
Although Ecuador, China, India, Indonesia, and the Philippines, for example, do not provide access to large distant water fleets, these low-income, food-deficit countries are major exporters of high-value fish products such as shrimp and demersal fish. As shown in the West African example, several countries in the region export high-value fish, which should provide a significant national economic gain so that cheaper forms of protein can be imported. In countries such as Ghana, however, the value of exports is often less than the value of imported fish, and the volume of imported fish does not meet the domestic demand for fish (Atta-Mills et al 2004).
Fish products are heavily traded, and exports from developing countries and the Southern Hemisphere presently offset much of the demand shortfall in European, North American, and Northeast Asian markets (Ahmed et al. 1999). Given the global extent of overfishing, however, it is likely that the global decline in marine fisheries landings, which already affects the poorer consumers in developing countries, will also catch up with consumers in industrial countries (Garcia and Newton 1997).
18.4.2 Unintentional Trade-offs
There are many unintended consequences of policies. However, it is worth noting that the economics of natural resources, including fisheries, usually only consider market forces and not the underlying biological constraints or environmental costs. For example, building an access road to a fishing village with the intention of connecting it to various cultural, social, and economic amenities (markets) has been shown in many cases to lead to massive increase in fishing effort and a collapse of local resources, the exploitation of which was previously regulated by the absorptive capacity of the local market.Fish aggregating devices, which are large floating structures, are constructed and use materials so that fish, especially pelagic fish, are attracted to them. They were seen as a cost-effective means to increase fish catches without affecting other aspects of the marine ecosystem or juvenile tuna stocks. However, monitoring of catches by these devices has shown that juvenile tunas are also attracted, which may, by increasing the effort on tuna juveniles and other ‘‘by-catch’’ species, have an impact on tuna stocks. Indeed, the mean size of caught fish is diminishing in both yellowfin tuna (T. albacares) and bigeye tuna (T. obesus) (Bromhead et al. 2003).Perverse incentives in fisheries are well studied by economists. They include size limits for landed fish, which encourage under- 498 Ecosystems and Human Well-being: Current State and Trends sized bycatch to be discarded at sea, and decommissioning schemes that lead to fleet modernization and ‘‘technology stuffing.’’ Alder and Lugten (2002) discuss this in the context of the EU Common Fisheries Policy.
18.5 Choices, Trade-offs, and Synergies with Other Systems
The ocean is the ultimate receptacle for discharges from the land and coast. While land use has significant and frequently obvious impacts on terrestrial and coastal systems, impacts on deeper marine systems are not as evident. However, indirect impacts such as air pollution deposition and water pollution are being detected in marine systems (such as the POPs found in high Arctic marine mammals). Marine systems play an important role in climate regulation, the water cycle, and coastal processes, especially in the recycling of nutrients. El Nin˜o events highlight the widespread geographic and ecosystem impact of perturbations of marine systems on pelagic fish stocks, coastal areas, and land systems.
18.5.1 Climate Change
Climate change is affecting the global distribution of heating and cooling of the ocean surface, which is a major factor in determining the large-scale patterns of ocean current flow and will have an impact on the marine ecosystem, including fisheries, in a number of ways (Mclean and Tsyban 2001).
Most marine fish and many other marine organisms have complex life cycles, featuring at least one planktonic early life stage. Consequently, reproductive habits and behaviors may be specifically adapted to current conditions, which will transport passively drifting larvae to appropriate nursery grounds. Bakun (1996) has shown that for the Brazilian sardine (Sardinella brasiliensis), enrichment of nearshore waters, the presence of a retention mechanism, and concentration of small fish in nearshore waters must all occur at the right place and time for successful recruitment. Climate change, acting through changes in sea temperature and especially wind patterns, will disturb and displace fisheries. Disruptions in current flow patterns in marine and estuarine systems, including changes to freshwater inputs as predicted under climate change, may cause great variations in reproductive success (Welch et al. 1998; Francis and Mantua 2003).
Research in the Northeast Pacific indicates that alternating climate ‘‘regimes’’ can affect marine ecosystem structure. Haigh et al. (2001) coupled a planktonic ecosystem model to a general ocean circulation model for the Pacific Ocean (18 S to the Bering Strait) to study the 1976 ‘‘regime shift.’’ The results showed clear geographical patterns in primary production and significant changes in the levels of primary production and standing stocks of phytoplankton and zooplankton before and after 1976. This implies that fish and other marine species will establish new geographic distributions as they seek new areas with the optimal temperature and food supplies. For example, it can be expected that North Pacific cold temperate species will move northward and will be replaced, along the coast of British Columbia, by warm temperate species (Welch et al. 1998). A similar set of replacements has been occurring in the last two decades in Europe, notably herring and pilchards in the Bay of Biscay, the English Channel, and the North Sea.
The capacity of fish species to adapt rapidly in reaction to such environmental changes is not clear at the present time. There are some hypothetical mechanisms available that might conceivably facilitate quite rapid adaptive adjustments (e.g., Bakun 2001). However, these adjustments imply high populations (biomass) ca- pable of producing numerous and varied offspring, some of which will be adapted to the newly opened niches. Also, oceanographic conditions may be changing too rapidly. Given the low biomasses for most exploited stocks, the prospect of rapid environmental change is doubly problematic for marine fisheries and marine biodiversity (Global marine biodiversity trends) preservation.
Finally, it should be noted that the changes in physical features predicted by various global and regional simulation models under likely CO2 emission scenarios fall well outside the parameter range so far observed under natural marine regimes (IPCC 2003).
18.5.2 Interactions with Coastal Systems
One of the major interactions between marine fisheries and coastal systems is through the life history of the large number of marine species that use coastal areas, especially estuaries, mangroves (Mangrove ecology), and seagrasses, as nurseries. (See [[Chapter 19 (Ecosystems and Human Well-Being: Volume 1: Current State and Trends: Marine Systems)]13].) Thus, coastal habitat modifications and coastal pollution, as well as inshore fishing, can adversely affect offshore fisheries by reducing the supply of recruits to the offshore adult stocks. The scale of this problem varies between continents, however. For example, a large fraction of the fish species occurring along the continental shelf of eastern North America produce juveniles that are dependent on estuarine habitats, while fish along the coasts of western Australia, northwestern Africa, or southwestern South America appear to depend less on coastal systems.
The presence of juveniles in coastal systems and of adults further offshore means that small-scale inshore and industrial offshore fisheries effectively compete for the same resources, even though they are apparently geographically separated.
18.5.3 Interactions with Island Systems
Small islands throughout the Pacific, Indian, and Atlantic Oceans have coastal and shelf areas whose physical and biological features differ from those typical of the biogeochemical province in which the islands are located. In many cases, these islands, whose nearshore fisheries have often reduced the limited coastal resources, are attempting to get access to pelagic offshore resources (see Chapter 23 (Ecosystems and Human Well-Being: Volume 1: Current State and Trends: Marine Systems)) (Petersen 2003). This has led to a new set of conflicts between island states and industrial countries with distant water fleets.
From the perspective of island states, the oceanic resource— even in its present depressed state—far exceeds the coastal fish population they initially relied on. The United Nations Convention on the Law of the Sea and various other treaties discussed later in this chapter have begun to provide mechanisms to address some of these issues of perception and equity.
18.6 User Rights and Protection Status of Marine Ecosystems
Marine systems are often described as ‘‘commons’’ (for everyone’s use) and their overexploitation as a tragedy (Hardin 1976). While this may hold true for the open ocean, complex property rights exist in many coastal areas. The property rights in question can be traditional (aboriginal), historical or local, and commercial (that is, the government sells the right to gain access to resources). The boundaries between these rights are frequently unclear and in some cases generate conflicts.
Some of the major groups of fishers involved in exploiting marine resources are described briefly in this section, along with major issues, outlooks, and prospects.
The introduction of UNCLOS and other jurisdictional restrictions has had minimal impact on countries or companies that can afford to ‘‘buy’’ access to fishing grounds. Other countries find themselves having to fish further offshore or purchase cheap, low-quality fish. This situation is clearly demonstrated in West Africa, where Europe has extended its fishing grounds from Northwest Africa to the whole of the West coast. Countries such as Nigeria and Ghana have seen a reduction in their traditional fishing grounds, intensification of small-scale fishing in coastal waters since they can not afford to gain access to foreign EEZs, and increases in imports of cheap, low-quality fish (Atta-Mills et al. 2004).
The term ‘‘aboriginal’’ refers to the descendents of the original inhabitants of countries whose population now largely consists of recent immigrants (such as in the Americas, Australia, and New Zealand). Aboriginal peoples are often marginalized, especially with regard to access to natural resources. In some areas, their existence is not even legally recognized. Other areas recognize aboriginal rights based on the original occupancy of the territory. Such rights are now recognized in Canada’s Constitution and Supreme Court decisions, for instance (Haggan and Brown 2002).
Similar rights were granted to New Zealand Maoris, who now own one third of the country’s fishing quotas. Examples of aboriginal groups with strong fishing traditions are the First Nations along the Pacific coast of Canada and the United States, the Maoris of New Zealand, and the Torres Strait Islanders of Northern Australia. In some situations, their rights can be a source of conflict with non-aboriginal fishers, whose behaviors and activities are governed by additional or different laws and regulations (Tomlinson 2003).
Aboriginal fishing methods tend to be used only in the coastal zone, but their impact can extend much farther, as the target species often have broad offshore distributions. Aboriginal fisheries are often subsistence fisheries, though not necessarily so. Indeed, the term ‘‘subsistence fisheries’’ is frequently a misnomer, since much of what is caught by subsistence fisheries is actually sold. True subsistence fisheries without subsequent sale of harvested products are now rare (an example is shellfish gathering in Kwazulu Natal, South Africa; see Branch et al. 2002).
Small-scale or artisanal fishers exist both in industrial and developing countries, and consequently their definition varies. However, competition between small-scale and large-scale fisheries is a common issue in all countries. Competition can be either direct (such as fixed gears versus trawlers targeting shrimps in tropical waters, as described in the next section) or indirect (such as coastal traps versus trawlers targeting different age classes of the same cod stock off Newfoundland). There is also competition for political and financial support as well as markets. The resulting conflicts, often couched as ‘‘equity’’ issues and aggravated by large-scale fleets, have tended to paralyze regulatory agencies in both industrial and developing countries. Community-based property rights may be one way of dealing with this issue, as seen in the Philippines, where local communities have jurisdiction over inshore waters within 15 kilometers of the coast (Rolden and Sievert 1993). However, synergies can also emerge with new markets, infrastructure improvements (processing and transport), and technology transfers occurring. Figure 18.18 illustrates the respective role and impacts of small-scale versus large-scale fisheries in one case study.
In most of the industrial world, such as the European Union and United States, the large-scale sector receives the bulk of government subsidies. Consequently, the economic efficiency of these fisheries is questionable. Another characteristic of large-scale fleets is that once local waters are depleted, they lead the expan- sion further abroad, often requesting subsidies to gain access to the resources of other countries, as with EU fleets off West Africa (Kaczynski and Fluharty 2002). However, the industrial fishing sector is declining as a source of employment in the industrial world. EU member countries and Japan, for example, are finding it difficult to recruit young workers into the industry and are turning to developing countries in Africa and Asia to crew their vessels (FAO 2002; Morales 1993).
Recreational fishing was considered relatively benign until recently, mainly because information about its impact has been limited. FAO’s first estimate of global recreational catches was put at only 0.5 million tons (Coates 1995), but recent estimates of over 1 million tons are probably more accurate (Coleman et al. 2004). Indeed, recent studies in British Columbia have shown that for inshore fisheries, the catch from the recreational sector can exceed the commercial sector (Forrest 2002). Recreational fishing is an important economic activity in some countries; in the United States it is worth approximately $21 billion a year; in Canada, $5.2 billion a year (Department of Fisheries and Oceans 2003); and in Australia, $1.3 billion a year (Henry and Lyle 2003). Although recreational fisheries often promote catch and release practices, the mortality associated with these practices is not well known and may be high (Wilde and Pope 2003).
There are major conflicts between commercial fishers, especially small-scale or artisanal ones, and recreational rod-and-line and underwater spear fishers in many countries (in Portugal, for instance). Large numbers of so-called recreational fishers in fact fish for a living and are not licensed or regulated in any way. In southern Europe, there is evidence that spear fishing has had a significant impact on inshore fish communities (to depths of 30–40 meters), with a decrease in abundance and mean size of many species, such as groupers and large sea breams. In addition, pressure on fish populations may have contributed to the disappearance of some small-scale fishing gear (such as small-hook longlines) due to the decline in catch rates and profitability (K. Erzini, Universidade do Algarve, personal communication 2004).
Marine tourism is a growing industry, principally in the marine wildlife tours sector. Similarly, coral reef (Coral reefs (collection)) (Coral reefs (collection)) tourism has increased in visitation levels and value, with a current net present value estimated at $9 billion (Cesar et al. 2004). The Great Barrier Reef attracts 1.6 million visitors each year and generates over $1 billion annually in direct revenue (Harriott 2002).
18.6.1 Competition between User Groups—Equity and Access Rights
Given the involvement of a number of user groups, management of fisheries and marine natural resource allocation requires the consideration of rights and equity among stakeholders. There is a strong tendency among fisheries economists to view individual transferable quotas and another form of ‘‘rights-based fishing’’ as the solution to mismanagement of fisheries (Hannesson 2000; Arnason and Gissurarson 1999). Others argue that the citizens of each country in question are the implicit owners of coastal and shelf fishery resources and that exploiting these resources can only be granted as a privilege for which payment is due, for example, through annual auctions (Macinko and Bromley 2002). The solution to these access and equity issues lies within the sociopolitical situation of each country rather than within the realm of science.
Shallow waters of tropical continental-shelf ecosystems are characterized by relatively high fish densities and the presence of high-value species, such as penaeid shrimps (Longhurst and Pauly 1987). The incursion of trawlers, which target shrimps for export, into shallow shelf ecosystems (10–100 meters) where artisanal fisheries operate results in open competition between the two fisheries for the same resource (Pauly 1997).Catching and discarding undersized fish is another source of conflict between the two fisheries. Shrimp trawl fisheries, particularly for tropical species, have been found to generate more discards than any other fishery type while accounting for small fraction of global marine fish landings (Kelleher 2004). Since many discarded species are of commercial value to small-scale fisheries, bitter conflicts between the two fisheries often ensue, leading to conflicts between fishers using different gear and political infighting over resource allocation and bycatch removal quotas (Alverson et al. 1994; Kelleher 2004; Pauly 1997).
Artisanal and industrial fisheries also experience conflicts in markets where they use the same inputs such as fuel and gear or they catch the same or similar species of fish (Panayotou 1982). Industrial fisheries may bid up the prices of fishing inputs or their massive landings may depress fish prices, making small-scale fishers increasingly uncompetitive. Industrial fisheries have access to low-interest institutional credit and loans and government subsidies, whereas small-scale fishers usually only have access to informal credit at interest rates many times higher than the institutional rates (Panayotou 1982).
Institutional support is often skewed in favor of the large-scale fishers because of their apparently higher efficiency and greater contribution to economic growth; their use of fewer landing areas, which allows economies of scale in the provision of infrastructure and the delivery of assistance programs; their political and economic power; and a general bias in favor of large-scale fisheries under an open-access regime. In contrast, small-scale fishers are geographically dispersed and lack political and economic power and hence do not generally benefit from institutional support (Bennett et al. 2001).
Several management interventions and conflict mitigation processes can minimize conflicts. These range from outright banning of certain gears—as occurred in western Indonesia in 1980, for example, when trawling was banned (Sardjono 1980)—to clarifying property rights and gear and area restrictions. In some cases, the role and recognition of traditional and local ecological knowledge are increasing as management responsibilities are transferred to local communities and stakeholders. For example, traditional and local knowledge is used to manage a bait fishery in the Solomon Islands (Johannes et al. 2000). Protection of the rights of small-scale fishers is also recognized explicitly in the Convention on Biological Diversity decision VII/5 and the FAO Code of Conduct for Responsible Fisheries.
18.6.2 Effectiveness of International Instruments in Managing Shared Stocks
While there are more than 100 fisheries access agreements (multilateral and bilateral) currently used to manage access to marine resources, few are monitored or evaluated for their effectiveness, equitable access, and sharing of economic benefits. The European Union has initiated a monitoring program for the EU’s Common Fisheries Policy, and other regional fisheries bodies are considering monitoring programs, but none have been developed to date (FAO 2001a).
A recent study of international fisheries instruments for the North Atlantic suggested that global and regional treaties covering the area are of limited effectiveness; the treaties themselves are not found to be weak, but many governments appear unwilling to act on commitments made under them (Alder and Lugten 2002).
The study showed that the 14 relevant instruments have a strong North-South gradient of decreasing implementation, as measured by adherence to reporting requirements, issuance of reports, and other formal requirements. On the other hand, there was no such gradient in the state of the stocks, and it could therefore be argued that these instruments are of limited effectiveness even when completely implemented. Consequently, reinforcing international agreements and ensuring they are implemented is likely to contribute significantly to solutions to the present problem of overfishing. This will require that these bodies free themselves from the influence (dominant participation) of the very industry they are intended to regulate, as documented in a detailed study of the U.S. Fisheries Management Councils (Okey 2003).
Various legal instruments, such as the Marine Mammal Protection Act in the United States, explicitly require protection of the food and habitat of charismatic marine fauna. International instruments such as the International Whaling Commission and the Convention on International Trade in Endangered Species of Wild Fauna and Flora restrict harvesting of species or limit bycatch.
A recent joint study between the CBD and the UNCLOS found that whereas the provisions of these two conventions are complementary and mutually supportive regarding the conservation and sustainable use of marine and coastal biodiversity, an important legal gap exists with respect to commercially oriented activities relating to marine genetic resources in areas beyond national jurisdiction. (Each country’s national legislation could address the issues within its EEZ.) The gap is yet to be addressed by the international community, and it would seem particularly important given the increasing importance of genetic resources in these areas and the threat posed to them by various activities, such as bio-prospecting by multinational pharmaceutical companies, that may be carried out without due regard to conservation and equity principles.
The implementation of North Atlantic agreements is among the best funded and supported administratively, and yet fisheries in the North Atlantic continue to decline (Alder and Lugten 2002). The refusal of EU-member governments to set sustainable harvest levels or to take a precautionary approach to the Common Fisheries Policy is a clear example of a well-funded management system supported by sound science with overfished stocks. Agreements in the South Atlantic, and for the most part in the rest of the world, are largely similar in nature to North Atlantic legal instruments. However, there are substantially fewer agreements elsewhere, and the level of support for them is much less, although some of the instruments of the southern polar biome are exceptions in terms of support. Globally, there is potential for instruments to assist in finding solutions to fisheries sustainability problems, but the lack of political commitment to their use to effect change prevents significant progress toward such solutions.
18.6.3 Effectiveness of Marine Protected Areas
Marine protected areas with no-take reserves at their core can reestablish the natural structures that have enabled earlier fisheries to maintain themselves. (See also [[Chapter 4 (Ecosystems and Human Well-Being: Volume 1: Current State and Trends: Marine Systems)]3].) MPAs are not a recent concept. Historically, many fisheries were sustained because a portion of the target population was not accessible. Most targeted fisheries were offshore or in areas adjacent to lands with low human populations and therefore subject to relatively low threat. However, modern fishing technology for mapping the seabed and for finding and preserving fish (artificial ice and blast freezing) expanded the reach of fishing fleets.
A number of recent studies have demonstrated that MPAs can help in managing fisheries (Roberts et al. 2002). Most of these studies have covered spatially small areas and primarily in tropical shelf systems, although emerging studies from temperate areas, such as New Zealand and Chile, have also demonstrated MPA effectiveness. However, other studies have found that MPAs have not delivered the expected benefits of protecting species and their habitats (Hilborn et al. 2004; Edgar and Barrett 1999; Willis et al. 2003). In many cases failure was due to either not including MPAs as part of a broader coastal management system or a lack of management effectiveness, funding, or enforcement. In the Gulf of Mexico, for example, the establishment of MPAs merely shifted fishing effort to other areas and increased the vulnerability of other stocks and endangered species (Coleman et al. 2004).
Knowledge on the size and location of MPAs that can act as effective buffers against the impacts of fishing requires further research. 502 Ecosystems and Human Well-being: Current State and Trends It has been widely and repeatedly demonstrated that marine protected areas, particularly no-take marine reserves, are essential to maintain and restore biodiversity in coastal and marine areas (COMPASS and NCEAS 2001). Their wide-scale adoption is inhibited by the perception that biodiversity is unimportant relative to fishers’ access to exploitable resources. Therefore, the proponents of marine reserves have been saddled with the additional task of demonstrating that setting up no-take reserves will increase fisheries yields in the surrounding areas, as well as determining the appropriate size and siting of marine reserves that are needed to at least sufficiently offset the loss of fishing grounds. This requirement, combined with initiatives by recreational fishers asserting rights to fish, has effectively blocked the creation of marine reserves in many parts of the world.
Thus while the cumulative area of marine protected areas is now about 1% of the world’s oceans, only about one tenth of that—0.1% of the world’s oceans—is effectively a no-take area. This gives an air of unreality to suggestions that 20% and an optimum of 30–50% of the world’s ocean should be protected from fishing to prevent the loss of some species now threatened with extinction and to maintain and rebuild some currently depleted commercial stocks (National Research Council 2001; Roberts et al. 2002; Airame et al. 2003; Agardy et al. 2003). Even the more modest CBD target of 10% MPA coverage by 2012 will be hard to reach.
One approach to resolving this dilemma is to take an adaptive management approach so that the use of MPAs within a suite of fisheries management options can be assessed and modified as new information emerges and lessons learned are shared (Hilborn et al. 2004). This avoids unrealistic expectations on the improved performance of MPAs. Any approach to the use of MPAs in managing marine ecosystems would also benefit enormously from including performance monitoring and enforcement programs to address some of the management problems that have traditionally hindered effectiveness (Coleman et al. 2004).
If properly located and within a context of controlled fishing capacity, no-take marine reserves enhance conventional fisheries management outcomes. They may, in some cases, reduce catches in the short term, but they should contribute significantly to improving fishers’ livelihoods as well as biodiversity over the mid to long term. Marine reserves generally perform this way in inshore shelf systems (such as reefs); many case studies, as shown in Saba Marine Park (Netherlands Antilles), Leigh Marine Reserve (New Zealand), and Sumilon Island Reserve (Philippines), are described in detail in Roberts and Hawkins (2000) to support this. However, understanding of the effectiveness of marine reserves in managing fisheries in deeper oceanic areas is more limited. Further, the protection and monitoring of these deep-sea areas and other undamaged areas may, in line with the precautionary principle, avoid the need for mitigation or restoration of the systems later, when costs are likely to be higher (and in some cases restoration may not be viable).
Already, the demand for fish resources has pushed fishing fleets into international waters, and as other resources become scarcer in national waters (such as gas, oil, minerals, and carbon sinks), conflicts over the best use of these common resources and spaces will increase. Hence the growing call for ocean zoning, including the creation of no-take zones that would reestablish the reserves that were once in place due to vessels lacking the technology to gain access to deeper, offshore areas, which in the past has protected exploited species.
18.6.4 Effectiveness of Fisher Mobilization
Recently, small-scale and artisanal fishers, with the assistance of organizations such as the International Collective in Support of Fishworkers, have established local groups to lobby government and industry. The mobilization of these groups is taking place globally in industrial and developing countries alike. In India (Mishra 1997), Chile (Phyne and Mansilla 2003), the Philippines, and Japan (Kurien 2004), for instance, fisher unions and collectives have used their numbers to lobby government and industry for improved resources access, better working conditions, and social benefits. In addition, these groups have provided mechanisms to enable women, who play a number of roles within the fishing sector (including sometimes also fish capture), to participate more in fisheries management. However, considerable resources and the legal basis to demand change are required, and in some countries mobilization of fishers is discouraged or hindered by governments or cultural norms (Alauddin and Hamid 1999).
18.7 Sustainability and Vulnerability of Marine Fisheries
Fishing has grown to become a much larger business than it was in the 1950s. Modern, highly capitalized fleets now range the oceans of the world, in some cases competing for a limited common resource with small-scale fishers and local communities.
Overcapitalization of the global fishing industry is, in fact, an enormous problem. FAO estimated that on a global basis world fisheries operated at an annual deficit of up to $20 billion in the 1990s (Milazzo 1998). Thus, present-day commercial fisheries actually represent a large net burden on other economic sectors. This unfortunate situation is at least to some degree due to the lack of a sound scientific basis to correctly gauge the productivity of the resources and to effectively manage impacts in the face of large-amplitude variability in both physical and biological aspects of ocean ecosystems.
In ecological terms, many marine fish appear to be vulnerable to anything above low levels of fishing pressure because of their biological characteristics, including their late age of sexual maturation, association with specialized or limited habitats that might also be vulnerable or widely damaged by fishing activities (such as trawling), and vulnerable life-history strategies such as aggregation spawning (Ehrhardt and Deleveaux 2001). Distinct genetic units may be susceptible to irrecoverable declines if they are self-recruiting or exhibit Allee-type effects (effects of birth rate declines at low population densities), whereby they cannot recover below some lower threshold of population numbers or density (Stephens and Sutherland 1999).
18.7.1 Natural Population Variability and Sustainability
Analysis of multidecadal time series fishery data has shown that many important fish populations are highly variable (e.g., Csirke and Sharp 1984; Schwartzlose et al. 1999). This is often in response to changes in ocean climate and ecosystem structure. This variability greatly compounds the difficulties in properly managing fisheries exploitation (Bakun and Broad 2002). For example, a level of fishing pressure calculated to be sustainable may cause unexpected collapse of a population whose productivity has decreased naturally. Moreover, even when evidence of declining numbers is available, the fishing industry may cite natural variability to argue against the results and question the need for action and may continue with unsustainable levels of exploitation.
Sustainability in fisheries, as evidenced by long-term data series showing no downward trend in catches, is generally a matter of fishers lacking either the technical means to catch more fish or the outlets (the large markets) to sell more than a small fraction of the fished population (Pauly et al. 2002). Increasing demand relative to the productivity of local resources and the technology to gain access to the entire distributional range of a species will tend to deplete the resource.
In a number of case studies, responses to fisheries management problems have mitigated or reversed the impact of fisheries. For instance, the introduction of community-based management of reef areas in the Philippines has resulted in increased fish landings that ultimately improved the well-being of those communities (Russ and Alcala 1994). Also, more effective enforcement measures for Namibian fisheries and the nationalization of the fishery sector contributed to better socioeconomic conditions for many coastal communities (Erastus 2002). In general, relatively small and often single-species fisheries can be restored, as has occurred in the Peruvian hake (Merluccius gayi peruanus) fishery (Instituto de Mar del Peru 2004).
However, there are also a number of spectacular failures—cod (Gadus morhua) in Newfoundland and orange roughy (Hoplostethus atlanticus) in New Zealand are two often-cited examples. A combination of increasing fishing efficiency through technology, expansion by Canadian fishers into fishing grounds previously used by foreign fleets, and an apparent but misleading increase in stock density as the overall biomass collapsed in the 1980s partly account for the repeated underestimation of the problem facing cod (Walters and Maguire 1996). Similarly, early in the development of orange roughy fisheries, there were management failures as the stocks were harvested at much higher rates than considered sustainable (Francis et al. 1995 in Clark 2001). Also, responses or interventions that mitigate or reverse negative effects of the largescale, multispecies fisheries, such as the demersal fisheries within EU waters, managed by the EU Common Fishery Policy have been implemented since the early 1970s. But these were ineffective due to lack of political will to decrease effort, poor data quality for specific fisheries and countries, and limited enforcement (Alder and Lugten 2002).
Some fisheries have been certified as sustainable by the Marine Stewardship Council and the Marine Aquarium Council. Only seven fisheries, including for Thames (U.K.) herring (Clupea harengus) and Western Australian rock lobster (Panulirus cygnus), have been MSC-certified since the program began in 1997, however, and most are small-scale with no impact at regional or global scales. Nevertheless, such schemes are important in communicating to consumers the need to manage fisheries sustainably and in educating them about the source and harvest techniques of the seafood products they purchase. Similarly, the Marine Aquarium Council’s certification scheme has had a positive impact on the aquarium trade (GEF 2004).
Conventions that have been effective in sustainably managing fisheries, such as the Pacific Salmon Treaty and the North Pacific Anadromous Fish Convention, have had small-scale impacts since they are focused on a particular species or set of species. Broader bodies, such as the International Commission for the Conservation of Atlantic Tunas, face greater challenges in managing the fisheries in their charge sustainably. Some fisheries, such as those under the EU’s Common Fisheries Policy and those in Canada and the United States (cod and Atlantic salmon (Salmo salar), for instance), have not been managed sustainably despite sound scientific evidence being available suggesting that reduced fishing effort would significantly assist the situation.
18.7.2 Thresholds
It has not so far been possible to predict the critical thresholds beyond which a fish stock will collapse, and the major stock col- lapses of the last few decades have been a surprise, even to those involved in monitoring and managing these stocks. One wellknown example is Newfoundland’s northern cod (G. morhua). Almost the same scenario was reenacted 10 years later, in 2001, in Iceland, which very nearly lost its cod stock (Marine Research Institute Reykjavik 2002), in spite of the Icelandic government’s commitment to sound fisheries management. Because of the unpredictability of these thresholds, precautionary approaches such as those involving marine protected areas and reductions in fishing effort (and in fishing mortality) are likely to safeguard against such thresholds being reached.
18.7.3 Areas of Rapid Change
Within 10–15 years of their arrival at a new fishing ground, new industrial fisheries usually reduce the biomass of the resources they exploit by an order of magnitude, (Myers and Worm 2003). This is well illustrated by the Gulf of Thailand demersal fisheries (Eiamsa-Ard and Amornchairojkul 1997), by orange roughy fisheries around various seamounts around New Zealand (Koslow et al. 2000), by the Antarctic fisheries discussed in [[Chapter 25 (Ecosystems and Human Well-Being: Volume 1: Current State and Trends: Marine Systems)]3], by the live reef fish trade (Sadovy et al. 2003b), and by a multitude of others. This process is often accelerated by encouragement from governments to ‘‘diversify’’ fisheries, often resulting in fleet overcapacity and a drive to exploit new or ‘‘unconventional’’ species. This level of biomass reduction renders the species in question extremely vulnerable to subsequent exploitation and other perturbations, notably those likely to result from climate change. Many areas of the coastal zone have undergone rapid changes directly due to coastal use and indirectly through upstream changes and land use. The consequences of this have been significant habitat loss, declining coastal environments, and reduced fish landings, as discussed in [[Chapter 19 (Ecosystems and Human Well-Being: Volume 1: Current State and Trends: Marine Systems)]14].
18.8 Management Interventions in Marine Systems
Any management initiative aiming to reduce the impacts of marine resource use or to strengthen sustainable use and conservation of biodiversity need to be addressed at different levels and by various means, and to involve local communities, including indigenous peoples. In the case of industrial fisheries, industry needs to be included as well. There are numerous examples from around the world of local involvement resulting in recovery of ecosystems and social benefits—the Philippines (Alcala and Russ 1994), Chile (Castilla 2000), and Brazil (Ferreira and Maida 2001), for example. There are also regional initiatives, as shown by the Regional Fishery Management Councils in the United States, that develop management plans to rebuild stocks. All sectors of society can be a part of the solution—governments by enforcing mandates and ensuring compliance with appropriate environmental codes, industry by operating responsibly, NGOs by providing capacity and training where needed, and consumers by demanding goods and services that are provided at minimal impact on marine ecosystems.
A number of international instruments can also be used to manage fishing and its impacts on the marine environment, domestically and internationally. Various fishing instruments have been discussed throughout this chapter. FAO’s Code of Conduct for Responsible Fisheries includes approaches for avoiding or mitigating the impacts of fishing on other components of ecosystems and is one of the few fisheries-specific instruments that includes fishing impacts on other species.
Other instruments, such as the Convention on International Trade in Endangered Species of Wild Fauna and Flora, can be used indirectly to manage the impact of fishing on threatened species through the development of species management strategies. CITES is an international agreement among 167 countries to cooperatively manage international trade in species of conservation concern, to ensure that trade does not threaten their survival in the wild.
The recent listing of seahorses and two other species of shark on CITES Appendix II now requires countries to determine that proposed exports will not be detrimental to the survival of the species in the wild (known as a nondetriment finding) as a precondition to permitting export. From a practical perspective, this means that countries that are parties to CITES need to implement management strategies to ensure that the export of seahorses and listed shark species (and therefore catch levels and methods) does not threaten the sustainability of their fisheries and wild populations.
The Convention on the Conservation of Migratory Species of Wild Animals (known as the Bonn Convention) and its Agreement on the Convention of Albatrosses and Petrels, as well as FAO’s International Plan of Action for Seabirds, are other examples of international management interventions in marine systems.
The continuation of present fisheries trends, including the buildup of fishing capacities, suggests a serious risk of losing more fisheries. Interventions such as the halting of destructive fishing practices by developing alternative technologies or financial incentives, reducing fishing effort, and establishing MPAs are needed to reverse the current trends. There is likely to be no single most suitable intervention; rather, a mix of interventions is likely to be the most effective approach. The composition of that mix will require an adaptive approach to management of marine ecosystems.
18.8.1 Integrating Management of Sectors in Marine Areas
No global or regional framework for integrated management of the oceans exists. Internationally, some activities on or in the high seas are managed through a range of organizations. For example, the International Seabed Authority manages seabed mining through UNCLOS, and the International Maritime Organization and the conventions it administers (such as the International Convention for the Prevention of Pollution from Ships, known as MARPOL) manages marine transportation and pollution, including dumping of waste at sea. These organizations have worked with industry on measures to reduce the impact of hazardous and damaging activities. Such measures have included, for example, the introduction of double-hulled oil tankers to lower the risk of oil spills. However, there is no integrated approach to managing ocean use, which has resulted in concern over some issues, such as bioprospecting, high-seas MPAs, and the management of marine biodiversity (Global marine biodiversity trends). Not all issues can be addressed within UNCLOS and changes to the convention or the introduction of a new legislative framework (including zoning plans) may be needed to overcome current impediments to making and managing the needed tradeoffs for equitable and sustainable use of ocean space and deep-sea resources.
National ocean policies based on sustainable development principles have been successful frameworks for integrating the management of the various marine sectors. Despite countries declaring their exclusive economic zones over the last 25 years, few countries have actually formulated or implemented comprehensive ocean policies. It is only in the last five years that we have seen the introduction of such policies in Australia, Canada, the United States, and the Netherlands (Alder and Ward 2001), along with, most recently, the Pacific Islands Regional Ocean Policy (South Pacific Regional Environment Program 2003).
UNEP has a comprehensive Regional Seas Programme that includes 13 regions and 140 countries with a focus on tackling the sources of degradation of marine and coastal systems. The program has a broad mandate and can integrate various sectors, including transportation and oil development, and initiatives at the regional scale to address pollution problems. It can also play a key role in establishing MPAs crossing multiple borders, as seen in the successful Mediterranean network of specially protected areas that conserve critical areas through MPAs, reserves, and refuges. The European Community’s marine strategy is an example of the challenges in managing marine ecosystems on a regional basis (EC 2002). Other regional programs, such as OSPAR for the North-East Atlantic and HELCOM for the Baltic, also integrate various marine sectors to address a range of marine issues.
18.8.2 Integrating Coastal Management and Ocean Policy
Oceanic and coastal ecosystems are tightly linked. While integrated coastal management is now well entrenched in many countries (see [[Chapter 19 (Ecosystems and Human Well-Being: Volume 1: Current State and Trends: Marine Systems)]15]), the development of ocean policies lags behind. Where ocean policies are in place, there is recognition of the need to take an ecosystem-based approach and to ensure that coastal management plans and ocean policies are harmonized. Depending on the legislative basis and jurisdictional issues, coastal management may be embedded within ocean policy, as demonstrated in Canada’s Oceans Act.
18.8.3 Marine Protected Areas
Attempting to maintain fisheries for depleted or collapsed fish populations (through subsidies, for example) is economically and ecologically damaging. As provided for in the 1982 Law of the Sea Convention, the 1995 UN Fish Stock Agreement, and the 1995 FAO Code of Conduct, declining populations must be rebuilt and marine ecosystem productivity must be restored as far as possible. No-take marine reserves can make an important contribution when they are part of an overall policy to maintain fisheries. No-take marine reserves are important for providing places where critical life stages, such as adult spawners and juveniles, can find refuge and for providing additional insurance to more conventional fisheries management while safeguarding against local extinctions.
Extinction is a gradual process, but many species of commercial fish species already have severely depleted populations. Avoiding the loss of threatened species must involve not only allowing their biomass to rebuild, but also rebuilding the ecosystems in which they live. MPAs may also contribute to reducing the impacts of global climate change by increasing biomass and widening age structure so that the population of fish stands a greater chance of withstanding wider fluctuations in the environment.
Moreover, the implementation of no-take marine reserves combined with other interventions, such as controls on fishing capacity, would be a more proactive response to fisheries management than current reactive approaches. Small reserves may be effective in protecting sedentary organisms, since they do not move or only move small distances. But marine reserves intended for the conservation or sustainable use of fish, marine mammals, seabirds, and large species need to be larger and particularly appropriately located to take into account the life characteristics of such species. Some species can spend various parts of their life cycle in different habitats (larval stages in estuaries, juvenile stages in coastal seagrass meadows, and adult stages in the open ocean, for instance), and marine reserves need to be strategically located to account for these differences. In areas left open for fishing, however, explicit consideration needs to be given to the food required to maintain recovered populations, and the scientific tools (such as ecosystem models) exist to perform the required accounting for biomass. Populations of small fish and invertebrates presently not exploited should not be viewed as a latent resource that should be developed. They are the remaining food basis for marine mammals, seabirds, and large fish that need to be sustained and rebuilt (as described in the next section).
The immediate and urgent need to manage risks to the marine biodiversity of seamounts and cold-water coral reefs through measures such as the elimination of destructive fishing practices, such as bottom trawling, has been highlighted in a number of recent international fora. These include the fourth meeting of the United Nations Open-ended Informal Consultative Process on Oceans and the Law of the Sea (2003), the 2003 World Parks Congress (recommendation 5.2.3 and the Congress document on emerging issues), the Eighth Meeting of the Convention on Biological Diversity Subsidiary Body on Scientific, Technical and Technological Advice (SBSTTA recommendation VII/5) (2003), the 2003 Defying Ocean’s End conference, the 10th Deep-Sea Biology Symposium (2003), and the 2nd International Symposium on Deep-Sea Corals (2003). These meetings have resulted in initiatives to protect cold-water reefs, as through a marine protected area established by Norway to conserve the Tisler Reef along the Norwegian-Swedish border.
18.8.4 Iron Enrichment of Ocean Waters for Carbon Sequestration and Increased Fish Yields
There is growing interest in experiments wherein large areas of low productivity oceanic waters are fertilized using micronutrients, principally iron (Boyd et al. 2000). One major reason for these experiments is to investigate the potential for sequestration of atmospheric carbon, whereby carbon is taken up by the cells of primary producers (the growth of marine phytoplankton populations is often limited by the availability of iron in the water), which would then sink to the bottom as marine snow (Cole 2001). The net effect of this process on carbon sequestration is not clear, however, because localized algal outbursts can also lead to anoxia and the production of methane, a powerful greenhouse gas.
It has been suggested that the enhanced primary production resulting from fertilization could also lead to increased fish yields and could even help alleviate dietary iron deficiencies in some parts of the world (Jones 2001). However, the success of such schemes is extremely doubtful, given the wide range of evolved adaptations that are required for planktivorous species such as anchovies to thrive in a highly productive variable environment, such as the Peruvian upwelling system (Bakun 1996).
18.8.5 Selected Examples of Human Responses to the Sustainability Challenge
While the problem of overfishing has been globally identified at high policy level as a key fisheries issue since the mid-1940s, the broad-scale recognition that it is also an important component of the global environment issue only emerged in the early 1990s in the wake of the U.N. Conference on Environment and Development (Garcia 1992). Even though the word ‘‘overfishing’’ was in common usage long before then (applying to ‘‘isolated’’ instances), there was still a general belief that the oceans had a an enormous capacity to provide fish and invertebrates for direct human consumption and for reduction products such as fishmeal and fish oils used in intensive food production systems (see, e.g., Idyll 1978; Pike and Spilhaus 1962).
In theory, the 1982 United Nations Convention on the Law of the Sea should have been an international instrument for wise use of the oceans: it espouses the right and need for coastal nations to monitor and manage their fish stocks. In retrospect, however, UNCLOS exacerbated overfishing problems in at least two important ways. First, as it gave coastal nations the ability to declare a 200-mile EEZ, many national governments saw this as an opportunity to greatly augment their fishing industries as sources of more secure employment and export earnings. While a few industrial countries managed to achieve some of the expected benefits by testing and adopting new management measures (such as limited entry and fishing rights), most others simply failed to realize them because of uncontrolled, anarchic, and subsidized development of chronic overcapacity (FAO 1993).
The subsidies injected into the industry in the 1980s and 1990s resulted in immense global overcapacity of fishing fleets, perhaps the most important problem—and apparently the hardest one to resolve—faced today in marine resource management (Mace 1997). For example, the EU’s subsidies for the construction of fishing vessels in the late 1980s and early 1990s resulted in overcapacity, and until recently attempts to reduce this capacity were not very effective (Alder and Lugten 2002). Subsidies in the form of employment insurance to Newfoundland fishers were one of the factors that contributed to overcapacity in the industry and the ultimate collapse of the cod fishery (Brubaker 2000).
Second, the UNCLOS requirement that coastal nations without sufficient fishing capacity to exploit the resources within their respective EEZs should make these resources available to other nations has ultimately proved detrimental to many developing nations. Although these countries do receive some form of reimbursement from other nations acquiring access, this has frequently resulted in payments that are significantly less than the value of the resource (particularly the long-term value; see Kaczynski and Fluharty 2002), in underreporting of foreign catches, in depletion of developing nations’ deep-sea resources, and in depletion of the coastal resources that supported local fishing communities.
The change in the perception that there was considerable potential to increase the exploitation levels of marine resources, including fisheries, which began in the early 1990s, was influenced by several factors: the globalization of information (making it more rapidly and more widely available), the awareness-raising work of the World Commission on Environment and Development and UNCED, and the active mobilization of the media by the NGO environmental movement. Fishing capacity was widely perceived to be getting out of hand. Opportunities for further expansion of fisheries were diminishing as an increasing portion of the globe was being surveyed and assessed, albeit yielding few new areas for fishing or new fisheries. Deepwater species were mostly found to have very low productivity. Many environmental organizations such as the World Wide Fund for Nature that had previously focused mainly activities on terrestrial systems and marine mammals began to expand into fisheries. Legislation was tightened in many nations, and work began on several binding and nonbinding international instruments, some related to ecosystems in general and others specifically on marine systems and fisheries.
Subsequently, there has been a marked increase in national and international instruments that are gradually changing people’s perceptions of the sustainability of current practices. For marine systems, the most important such instruments range from the 1992 Rio Declarations formulated at UNCED to several FAO International Plans of Action, including the International Plan of Action for the Management of Fishing Capacity (FAO 2001b) and the International Plan of Action to Deter, Prevent and Eliminate Illegal, Unreported and Unregulated Fishing (FAO 2001b). (See also MA, Policy Responses, Chapter 6.) All of these embody some facet of the ‘‘precautionary approach,’’ which has been instrumental in shifting attention to the benefits of conservative harvest strategies rather than risk-prone management (Mace 2001).
Concerted efforts to implement these agreements have been launched in many regions, with results ranging from unanticipated levels of success, to moderate success, and to failure. In terms of successes, the most notable accomplishments appear to have been those where national legislations have been modified to better accomplish the spirit and intent of the international instruments. For example, the most recent amendment to the Magnuson- Stevens Fisheries Conservation and Management Act in the United States (popularly known as the Sustainable Fisheries Act) embodied the precautionary approach (without mentioning it by name) as exemplified in Annex 2 on the implementation of UNCLOS (UN 1995), in which the fishing mortality associated with maximum sustainable yield is suggested as a limit reference point to be avoided rather than a target that is routinely exceeded.
This has resulted in considerable reductions in fishing mortality for some previously overfished U.S. fisheries, and several have rebuilt to levels not recorded in three or more decades.
One of the most dramatic examples is the density of Georges Bank scallops, which increased eighteenfold in the seven years following exceptional recruitment and implementation of management measures for bottom fishing that resulted in substantial reductions in fishing mortality during the fishing closure. Other U.S. examples include mid-Atlantic scallops (Haliotis spp), Georges Bank haddock (Melanogrammus aeglefinus), Georges Bank yellowtail flounder (Limanda ferruginea), Atlantic stripped bass (Morone saxatilis), Atlantic Acadian redfish (Sebastes fascicatus), Pacific chub mackerel (Scomber japonicus), and Pacific sardine (Sardinops sagax) (Murawski et al. 2000; NEFSC 2002). Yet while these and many other stocks in the United States and elsewhere are slowly but steadily rebuilding (NMFS 2004), the biomass of most of them is generally still well below historic levels. Closures of seamount fisheries on the Chatam Rise in New Zealand have also resulted in the slow rebuilding of orange roughy biomass (Clark 2001). Globally, however, these success stories represent a small proportion of the many overfished stocks.
Other examples of successful national initiatives include Australia’s implementation of the Environment Protection and Biodiversity Act in 1999, which requires several fisheries to be assessed for their ecological sustainability. Regionally, the ICCAT has been able to work toward compliance of management measures for nonmember countries through tuna import and export restrictions by member countries.
Notable failures in fisheries management are exemplified by attempts to reduce harvest overcapacity while maintaining that exploitation of natural resources is a human right. This ‘‘birthright’’ is hard to dispute: all humans ought to have the ‘‘right’’ to a healthy life with adequate nutrition. And for some people, fish are one of the few available sources of protein. Solutions to this dilemma lie in assistance that improves the health, education, and non-fisheries-related employment opportunities of coastal communities as well as empowering coastal communities to manage resources sustainably.
Even relatively rich industrial nations that routinely pay their farmers not to produce crops seem unable to resolve the fish harvesting overcapacity problem, however. The European Union, for example, appears to have been largely unsuccessful with the capacity reduction plan in its Common Fisheries Policy, which has been in place for more than 15 years. With a few exceptions, substantial overcapacity reductions have been recorded in cases where individual transferable quotas, which allocated a quota of the catch to license holders as well as allowing those individuals to sell part or all of their quota, have been implemented (for instance, ITQs in New Zealand, Iceland, and some U.S. fisheries, such as Atlantic surf clams (Spisula solidissima) and ocean quahogs (Arctica islandica), South Atlantic wreckfish (Polyprion americanus), and Pacific halibut (Hippoglossus stenolepis) and sablefish (Anoplopoma fimbria)).
Although the emphasis in recent years has been on unsustainable fishing practices, fisheries represent only one of many human influences on marine ecosystems. In coastal marine systems in particular, coastal development—with concomitant problems of local pollution and habitat destruction—is very important. (See [[Chapter 19 (Ecosystems and Human Well-Being: Volume 1: Current State and Trends: Marine Systems)]16].) Non-fisheries human influences such as marine debris and oil slicks are also important on the high seas. As a result, as described earlier, several nations are attempting to develop legislation and policies to facilitate integrated management of marine systems—that is, coordinated management of all alternative uses of the ocean. Such uses include harvesting marine species for food and other purposes, aquaculture, research, oil and gas exploration, ocean mining, dredging, ocean dumping, energy generation, ecotourism, marine transportation, and defense. To date, it has proved difficult to integrate the management of all these activities because the authorities regulating these activities are usually independent of one another (Sissenwine and Mace 2003).
In Australia, the Environment Protection and Biodiversity Act requires management agencies to demonstrate that the fishery or fisheries are ecologically sustainable, with defined benchmarks to quantitatively assess sustainability. Since the introduction of this Act in 1999, more than 35 fisheries have been assessed (www.ea .gov.au/coasts/fisheries/assessment/index.html). The private sector has responded similarly to the introduction of the Marine Stewardship Council’s accreditation scheme for fisheries, which is supported by major fish buyers, such as Unilever, and conservation organizations, such as WWF.
With increased public awareness of the limits of marine systems, binding and nonbinding international instruments in place, tightened national legislations, and a few success stories that emphasize the positive benefits of conservation, the future of the oceans may appear brighter than it did a decade ago. However, this is not the time for complacency, particularly considering the likely increase in pressure on natural resources that will result from the world’s growing human population and rising incomes.
References
- Abella, A.J., J.F. Caddy, and F. Serena, 1997: Do natural mortality and availability decline with age? An alternative yield paradigm for juveniles fisheries, illustrated by the hake Merluccius merluccius fishery in the Mediterranean. Aquatic Living Resources, 10, 257–269.
- Agardy, T., P. Bridgewater, M.P. Crosby, J. Day, P.K. Dayton, R. et al., 2003: Dangerous targets? Unresolved issues and ideological clashes around marine protected areas. Aquatic Conservation-Marine and Freshwater Ecosystems, 13(4), 353–367.
- Ahmed, M., C. Delgado, and S. Sverdrup-Jensen, 1999: The growing need for fisheries policy research in developing countries. In: Fisheries Policy research in developing countries: issues, priorities and needs, M. Ahmed, C. Delgado, S. Sverdrup- Jensen, and R.A.V. Santos (eds.). ICLARM Conference Proceedings 60, 1–4.
- Airame, S., J.E. Dugan, K.D. Lafferty, H. Leslie, D.A. McArdle, and R.R. Warner, 2003: Applying ecological criteria to marine reserve design: A case study from the California Channel Islands. Ecological Applications, 13(1), S170–S184.
- Alauddin, M. and M.A. Hamid, 1999: Shrimp culture in Bangladesh with emphasis on social and economic aspects. In: Towards Sustainable Shrimp Culture in Thailand and the Region, P.T. Smith (ed.), Australian Centre for International Agricultural Research (ACIAR), Canberra (Australia), 53–62.
- Alcala, A.C. and G.R. Russ, 1994: Sumilon Island Reserve: 20 years of hopes and frustrations. NAGA, The ICLARM Quarterly, 17(3), 8–12.
- Alder, J. and L. Christanty, 1998: Taka Bonerate: Developing a strategy for community-based management of marine resources. In: Living through Histories. Culture, History and Social Life in South Sulawesi, K. Robinson and M. Paeni (eds.), Department of Anthropology, Research School of Pacific and Asian Studies, the Asutralian National University, Canberra (Australia). Published in association with The National Archives of Indonesia (Arsip Nasional Republik Indonesia), 229–248.
- Alder, J. and T. Ward., 2001: Australia’s ocean policy—sink or swim? Journal of Environment and Development, 10, 266–289.
- Alder, J. and G. Lugten, 2002: Frozen fish block: how committed are North Atlantic States to accountability, conservation and management of fisheries? Marine Policy, 26(5), 345–357.
- Alder, J. and R.U. Sumaila, 2004: Western Africa: a fish basket of Europe past and present. Journal of Environment and Development, 13, 156–178.
- Alder, J. and R. Watson, in press: Globalization and its effects on fisheries. In: Proceedings of the Workshop Globalization: Effects on fisheries, 12–14 August 2004, Quebec (Canada), W. Taylor, M. Schechter, and L. Wolfson (eds.), Cambridge University Press, NY (USA).
- Alverson, D.L., M.H. Freeberg, J.G. Pope, and S.A. Murawski, 1994: A Global Assessment of Fisheries Bycatch and Discards. FAO Fisheries Technical Paper 339, Food and Agriculture Organization of the United Nations (FAO), Rome (Italy), 233 pp.
- Anonymous, 2002: Patterns and propensities in Greek fishing effort and catches. Report to the EU (DGXIV) Project 00/018, European Commission, Brussels (Belgium).
- Archer, F., T. Gerrodette, and A. Jackson, 2002: Preliminary estimates of the annual number of sets, number of dolphins chased, and number of dolphins captured by stock in the tuna purse-seine fishery in the eastern tropical Pacific, 1971—2000. Southwest Fisheries Science Center (SWFSC), National Marine Fisheries Service, NOAA, La Jolla, CA (USA), 26 pp.
- Arnason, R. and H. Gissurarson (eds.), 1999: Individual Transferable Quotas in Theory and Practice: Papers Exploring and Assessing the Radical Reorganization of Ocean Fisheries in the Final Decades of the 20th Century. The University of Iceland Press, Reykjavik (Iceland).
- Aronson, R.B. and D.B. Blake, 2001: Global climate change and the origin of modern benthic communities in Antarctica. American Zoologist, 41, 27–39. Atta-Mills, J., U.R. Sumaila, and J. Alder, 2004: The decline of a regional fishing nation: The case of Ghana and West Africa. Natural Resources Forum, 28(1), 13–21.
- Baker, C.M., B.J. Bett, D.S.M. Billett, and A.D. Rogers, 2001: An environmental perspective. In: The status of natural resources on the high seas, WWF/ IUCN (ed.), WWF/IUCN, Gland (Switzerland), 1–67.
- Bakun, A., 1996: Patterns in the Ocean: Ocean Processes and Marine Population Dynamics. California Sea Grant College System, University of California, La Jolla, CA (USA), 323 pp.
- Bakun, A., 2001: ‘School-mix feedback’: a different way to think about low frequency variability in large mobile fish populations. Progress in Oceanography, 49(1–4), 485–511.
- Bakun, A. and K. Broad, 2003: Environmental ‘loopholes’ and fish population dynamics: comparative pattern recognition with focus on El Nin˜o effects in the Pacific. Fisheries Oceanography, 12:458–473.
- Barrett, P., 2003: Palaeoclimatology—Cooling a continent. Nature, 421(6920), 221–223.
- Beaugrand, G. and P.C. Reid, 2003: Long-term changes in phytoplankton, zooplankton and salmon related to climate. Global Change Biology, 9, 801– 817.
- Beaugrand, G., K.M. Brander, J.A. Lindley, S. Souissi, and P.C. Reid, 2003: Plankton effect on cod recruitment in the North Sea. Nature, 426(6967), 661–664.
- Bennett, E., A. Neiland, E. Anang, P. Bannerman, A.A. Rahman, et al. 2001: Towards a better understanding of conflict management in tropical fisheries: evidence from Ghana, Bangladesh and the Caribbean. CEMARE Research Paper 159, Centre for the Economics and Management of Aquatic Resources (CEMPAGE ARE), Department of Economics, University of Portsmouth, Portsmouth (UK), 22 pp.
- Birkeland, C. and A. Friedlander, 2002: The importance of refuges for reef fish replenishment in Hawai’i. Hawai’i Audubon Society, Honolulu, HI (USA), 19 pp.
- Bjørndal, T. 1988: The Optimal Management of North Sea Herring, Journal of Environmental Economics and Management, 15, 9–29.
- Bonnell, M.L. and R.K. Selander, 1974: Elephant Seals—Genetic-Variation and near Extinction. Science, 184(4139), 908–909.
- Bopp, L., P. Monfray, O. Aumont, J.L. Dufresne, H. Le Treut, G. Madec, L. Terray, and J.C. Orr, 2001: Potential impact of climate change on marine export production. Global Biogeochemical Cycles, 15(1), 81–99.
- Boyd, P.W., A.J. Watson, C.S. Law, E.R. Abraham, T. Trull, et al. 2000: A mesoscale phytoplankton bloom in the polar Southern Ocean stimulated by iron fertilization. Nature, 407(6805), 695–702.
- Branch, G.M., J. May, B. Roberts, E. Russell, and B.M. Clark, 2002: Case studies on the socio-economic characteristics and lifestyles of subsistence and informal fishers in South Africa. South African Journal of Marine Science- Suid-Afrikaanse Tydskrif Vir Seewetenskap, 24, 439–462.
- Bromhead, D., J. Foster, R. Attard, J. Findlay, and J. Kalish, 2003: A review of the impact of fish aggregating devices (FADs) on tuna fisheries: final report to Fisheries Resources Research Fund. Bureau of Rural Sciences (BRS), Canberra (Australia), 122 pp.
- Brownell, R.L.J. and A.V. Yablokov, 2002: Illegal and pirate whaling. In: Encyclopedia of Marine Mammals, W.F. Perrin, B. Wu¨ rsig, and J.G.M. Thewissen (eds.), Academic Press, San Diego, CA (USA), 615–617.
- Brubaker, E., 2000: Unnatural Disaster: How Politics Destroyed Canada’s Atlantic Groundfisheries. In Political Environmentalism, Anderson, T. (ed.), Hoover Institution Press, Stanford (USA). Cited December 2004, Available at http://www.environmentprobe.org/enviroprobe/pubs/ev551.htm
- Bryant, D., L. Burke, J.W. McManus, and M. Spalding, 1998: Reefs at Risk: A map based indicator of threats to the world’s coral reefs. World Resources Institute (WRI), Washington, D.C. (USA), 56 pp.
- Burke, L., Y. Kura, K. Kassem, C. Ravenga, M. Spalding, and D. McAllister, 2001: Pilot Assessment of Global Ecosystems: Coastal Ecosystems. World Resources Institute (WRI), Washington, D.C. (USA), 94 pp.
- Caddy, J.F., 1990: Options for the regulation of Mediterranean Demersal fisheries. Natural Resource Modelling, 4(4), 427–475.
- Caddy, J.F., 1993: Towards a comparative evaluation of human impacts on fishery ecosystem of enclosed and semi-enclosed seas. Review of Fisheries Science, 1(1), 57–95.
- Caddy, J.F., 1998: Issues in Mediterranean Fisheries Management: Geographical Units and Effort Control. Studies and Reviews No. 70. General Fisheries Council for the Mediterranean, Food and Agriculture Organization of the United Nations (FAO), Rome (Italy), 63 pp.
- Caddy, J.F., R. Refk, and T. Dochi, 1995: Productivity Estimates for the Mediterranean— Evidence of Accelerating Ecological Change. Ocean & Coastal Management, 26(1), 1–18.
- Carlton, J.T., J.B. Geller, M.L. Reaka-Kudla, and E.A. Norse, 1999: Historical extinctions in the sea. Annual Review of Ecology and Systematics, 30, 515–538.
- Castilla, J.C., 2000: Roles of experimental marine ecology in coastal management and conservation. Journal of Experimental Marine Biology and Ecology, 250(1–2), 3–21.
- Castillo, S. and J. Mendo, 1987: Estimation of Unregistered Peruvian Anchoveta (Engraulis ringens) in Official Catch Statistics, 1951–1982. In: The Peruvian Anchoeta and Its Upwelling Ecosystem: Three Decades of Change, D. Pauly and I. Tsukayama (eds.), ICLARM, Manilla (Philippines), 109–116. CCAMLR, 2002: Statistical Bulletin. Commission for the Conservation of Antarctic Marine Living Resources (CCAMLR), Hobart (Australia), 155 pp.
- Cesar, H., L. Burke, and L. Pet-Soede, 2003: The Economics of Worldwide Coral Reef Degradation. ICRAN, Cambridge (UK) and WWF Netherlands, Zeist (Netherlands).
- Chavez, F.P., P.G. Strutton, C.E. Friederich, R.A. Feely, G.C. Feldman, et al. 1999: Biological and chemical response of the equatorial Pacific Ocean to the 1997–98 El Nino. Science, 286(5447), 2126–2131.
- Christensen, V. and D. Pauly, 2001: Coral reef and other tropical fisheries. In: Encyclopedia of Ocean Sciences, J.H. Steele, S.A. Thorpe, and K.K. Turekian (eds.). 1, Academic Press, San Diego, CA (USA), 534–538.
- Christensen, V., S. Gue´nette, J.J. Heymans, C.J. Walters, R. Watson, D. Zeller, and D. Pauly, 2003: Hundred-year decline of North Atlantic predatory fishes. Fish and Fisheries, 4(1), 1–24.
- Christy, F.T., 1997: Economic Waste in Fisheries: Impediments to Change and Conditions for Improvement. In: Proceedings from the 20th American Fisheries Society Symposium: Global Trends—Fisheries Management, 14–16 June 1994, 508 Ecosystems and Human Well-being: Current State and Trends Seattle, WA (USA), E.K. Pikitch, D.D. Huppert, and M.P. Sissenwine (eds.), American Fisheries Society, Bethesda, MD (USA), 28–39.
- Clapham, P.J., S.B. Young, and R.L. Brownell, 1999: Baleen whales: conservation issues and the status of the most endangered populations. Mammal Review, 29(1), 35–60.
- Clark, C.W., 1973: Economics of Overexploitation. Science, 181(4100), 630– 634.
- Clark, M., 2001: Are deepwater fisheries sustainable?—the example of orange roughy (Hoplostethus atlanticus) in New Zealand. Fisheries Research, 51(2–3), 123–135.
- Coates, D., 1995: Inland capture fisheries and enhancement: status, constraints and prospects for food security. Paper presented at the International Conference on sustainable Contribution of Fisheries to Food Security. Organized by the Government of Japan in collaboration with the Food and Agriculture Organization of the United Nations (FAO), Kyoto (Japan), 82 pp.
- Cole, K., 2001: Open Ocean iron Fertilization for Scientific Study and Carbon Sequestration. Paper presented at the First National Conference on Carbon Sequestration, 14–17 May. NETL Publications, Washington, D.C. (USA).
- Coleman, F.C., P.B. Baker, and C.C. Koenig, 2004: A review of Gulf of Mexico marine protected areas: Successes, failures, and lessons learned. Fisheries, 29(2), 10–21.
- COMPASS and NCEAS (National Center for Ecological Analysis and Synthesis), 2001: Scientific Consensus Statement on Marine Reserves and Marine Protected Areas. Cited December 2003. Available at http://www.nceas.ucsb .edu/Consensus
- Constable, A.J., W.K. de la Mare, D.J. Agnew, I. Everson, and D. Miller, 2000: Managing fisheries to conserve the Antarctic marine ecosystem: practical implementation of the Convention on the Conservation of Antarctic Marine Living Resources (CCAMLR). Ices Journal of Marine Science, 57(3), 778–791.
- Csirke, J. and G.D. Sharp, (eds), 1984: Reports of the Expert Consultation to Examine the Changes in Abundance and Species Composition of Neritic Fish Resources, San Jose, Costa Rica, 18–29 April 1983. FAO Fish. Rep. Ser. No.291 (1). FAO, Rome (Italy), 102 pp.
- Delgado, C.L., M. Rosegrant, H. Steinfeld, S. Ehui, and C. Courbois, 1999: Livestock to 2020: The Next Food Revolution. Food, Agriculture, and the Environment Discussion Paper 28, International Food Policy Research Institute, Washington, D.C. (USA); Fisheries and Agriculture Organization of the UNited Nations (FAO), Rome (Italy); International Livestock Research Institute, Nairobi (Kenya), 83 pp.
- Delgado, C.L., N. Wada, M.W. Rosegrant, S. Meijer, and M. Ahmed, 2003: Fish to 2020: Supply and Demand in Changing Global Markets. International Food Policy Research Institute (IFPRI), Washington, D.C. (USA) and WorldFish Center, Penang (Malaysia), 226 pp.
- Denney, N.H., S. Jennings, and J.D. Reynolds, 2002: Life-history correlates of maximum population growth rates in marine fishes. Proceedings of the Royal Society of London Series B-Biological Sciences, 269(1506), 2229–2237.
- DFO (Fisheries and Oceans Canada), 2004: 2000 Survey of Recreational Fishing in Canada. [online] Cited November 2004. Available at http://www.dfo -mpo.gc.ca/communic/statistics/recreational/canada/2000/index_e.htm.
- Duhamel, G. and J. Claudet, 2002: Preliminary analysis on the Kerguelen shelf icefish Champsocephalus gunnari stock from 1996/97 to 2000/01: no evidence in the recovery. WG-FSA-02/65, Commission for the Conservation of Antarctic Marine Living Resources (CCAMLR), Hobart (Australia).
- Dulvey, N.K., Y. Sadovy, and J.D. Reynolds, 2003: Extinction vulnerability in marine populations. Fish and Fisheries, 4(1), 25–64.
- EC (European Commission), 2002: Towards a strategy to protect and conserve the marine environment. Communication from the Commission to the Council and the European Parliament, Brussels (Belgium), 35 pp.
- Edgar, G.J. and N.S. Barrett, 1999: Effects of the declaration of marine reserves on Tasmanian reef fishes, invertebrates and plants. Journal of Experimental Marine Biology and Ecology, 242(1), 107–144.
- Ehrhardt, N.M. and V. Deleveaux 2001: Report on the 199–2001 Nassau grouper stock assessments in the Bahamas. Government of the Bahamas, Nassau.
- Eiamsa-Ard, M. and S. Amornchairojkul, 1997: The marine fisheries of Thailand, with emphasis on the Gulf of Thailand trawl fishery. In: Status and Management of tropical coastal fisheries in Asia, G. Silvestre and D. Pauly (eds.), ICLARM Conference Proceedings 53, 85–95.
- Erastus, A.N., 2002: The Development of the Namibianisation Policy in the Hake Subsector, 1994–1999. NEPRU Working Paper No. 82, The Namibian Economic Policy Research Unit (NEPRU), Windhoek, Namibia.
- Erdmann, M.V. and L. Pet-Soede, 1996: How fresh is too fresh? The live reef food fish trade in Eastern Indonesia. NAGA, The ICLARM Quarterly, 19(1), 4–8.
- FAO (Food and Agriculture Organization of the United Nations), 1981: Atlas of the living resources of the seas. Fisheries and Agriculture Organization of the United Nations, Rome (Italy), 23 pp and 73 maps.
- FAO, 1993: Marine Fisheries and the Law of the Sea: a decade of change. A special chapter (revised) of the State of Food and Agriculture. Fisheries Circular No. 853, Food and Agriculture Organization of the United Nations, Rome (Italy), 66 pp.
- FAO, 2001a: Report of the Second Meeting of FAO and Non-FAO Regional Fishery Bodies or Arrangements, 20–21 February. FAO Fisheries Report No. 645, Food and Agriculture Organization of the United Nations, Rome (Italy).
- FAO, 2001b: International Plan of Action to Prevent, Deter and Eliminate Illegal, Unreported and Unregulated Fishing. Food and Agriculture Organization of the United Nations, Rome (Italy).
- FAO, 2002: The State of World Fisheries and Aquaculture 2002. Food and Agriculture Organization of the United Nations, Rome (Italy).
- FAO, 2003: FAOSTAT2 Statistics Database. [online] Food and Agriculture Organization of the United Nations. Cited November 2004.
- FAO, n.d.: Number of fishers doubled since 1970. [online] Food and Agricultural Organization of the United Nations. Cited 30 November 2003. Available at http://www.fao.org/fi/highligh/fisher/c929.asp. Ferreira, B.P. and M. Maida, 2001: Fishing and the Future of Brazil’s Northeastern Reefs. InterCoast, 38, 22–23.
- Fiorentini, L., J.F. Caddy, and J.I. de Leiva, 1997: Long and short-term trends of Mediterranean fishery resources. Studies and Reviews. General Fisheries Council for the Mediterranean no. 69, Food and Agriculture Organization of the United Nations (FAO), Rome (Italy), 72 pp.
- Forrest, R., 2002: Estimating the sports catch in northern BC. In: Restoring the past to salvage the future: report on a community participation workshop in Prince Rupert, BC, T.J. Pitcher, M.D. Power, and L. Wood (eds.), Fisheries Centre, University of British Columbia (UBC), Vancouver (Canada), Fisheries Centre Research Reports 10(7)38–40.
- Francis, R.I.C.C., M.R. Clark, R.P. Coburn, K.D. Field, and P.J. Grimes, 1995: Assessment of the ORH 3B orange roughy fishery for the 1994 1995 fishing year. NZ Fish. Assess. Res. Doc. 95/4, National Institute of Water & Atmospheric Research (NIWA), Wellington (New Zealand), 43 pp.
- Francis, R.C. and N.J. Mantua, 2003: Climatic influences on salmon populations in the NE Pacific. In Assessing Extinction Risk for West Coast Salmon. NMFS-NWFSC-56. Seattle, USA, 37–76pp.
- Freiwald, A., J.H. Fossa°, A. Grehan, T. Koslow, and J.M. Roberts, 2004: Coldwater coral reefs. Out of sight—no longer out of mind. UNEP-WCMC, Cambridge (UK), 86 pp.
- FSBI (Fisheries Society of the British Isles), 2004: Effects of fishing on biodiversity in the North Sea. Briefing Paper 3, Cambridge (UK), 13 pp.
- Gandini, P., P.D. Boersma, E. Frere, M. Gandini, T. Holik, and V. Lichtschein, 1994: Magellanic Penguins (Spheniscus-Magellanicus) Affected by Chronic Petroleum Pollution Along Coast of Chubut, Argentina. Auk, 111(1), 20–27.
- Garcia, S.M., 1992: Ocean fisheries management. The FAO programme. In: Ocean management in global change, P. Fabbri (ed.), Elsevier Applied Science, 381–418.
- Garcia, S.M. and C. Newton, 1997: Current Situation, trends and prospects in world capture fisheries. In: Proceedings of the 20th American Fisheries Society Symposium: Global Trends -Fisheries Management, 14–16 June 1994, Seattle, WA (USA), E.K. Pikitich, D.D. Huppert, and M. Sissenwine (eds.), American Fisheries Society, Bethesda, MD (USA), 3–27. GEF (Global Environment Facility), 2004: Philippines and Indonesia: Marine Aquarium Market Transformation Initiative (MAMTI). TheWorld Bank, Jakarta, Indonesia, 172 pp.
- Gerrodette, T., 2002: Tuna-Dolphin issue. In: Encyclopedia of marine mammals, W.F. Perrin, B. Wu¨rsig, and J.G.M. Thewissen (eds.), Academic Press, San Diego, CA (USA), 1269–1273.
- Gjøsaeter, J. and K. Kawaguchi, 1980: A review of the world resources of mesopelagic fish. FAO Fisheries Technical Paper 193, Fisheries and Agriculture Organization of the United Nations, Rome (Italy), 151 pp.
- Glover, A.G. and C.R. Smith, 2003: The deep-sea floor ecosystem: current status and prospects of anthropogenic change by the year 2025. Environmental Conservation, 30(3), 219–241.
- Gray, J.S., 2002: Species richness of marine soft sediments. Marine Ecology Progress Series, 244, 285–297. Haggan, N. and P. Brown, 2002: Aboriginal fisheries issues: the west coast of Canada as a case study. In: Production systems in fishery management, D. Pauly and M.L.D. Palomares (eds.), Fisheries Centre, University of British Colum- Marine Fisheries Systems 509 bia (UBC), Vancouver (Canada), Fisheries Centre Research Reports 10(8) 17–21.
- Haigh, S.P., K.L. Denman, and W.W. Hsieh, 2001: Simulation of the planktonic ecosystem response to pre- and post-1976 forcing in an isopycnic model of the North Pacific. Canadian Journal of Fisheries and Aquatic Sciences, 58(4), 703–722.
- Hall, M.A., D.L. Alverson, and K.I. Metuzals, 2000: Bt-Catch: problems and solutions. Marine Pollution Bulletin, 41:204–219.
- Hannesson, R., 2000: A note on ITQs and optimal investment. Journal of Environmental Economics and Management, 40(2), 181–188.
- Hardin, G., 1976: Naturalist at Large—Fishing Commons. Natural History, 85(7), 9-&.
- Harriott, V.J., 2002: Marine tourism impacts and their management on the Great Barrier Reef. CRC Reef Research Centre Technical Report No. 46, CRC Reef Research Centre, Townsville (Australia), 41 pp.
- Heal, G.M., 1998: Valuing the Future: Economic Theory and Sustainability. Columbia University Press, New York, NY (USA), 224 pp.
- Henry, G.W. and M. Lyle, 2003: The national recreational and indigenous fishing survey. FRDC Project No. 99/158, Australian Government Department of Agriculture, Fisheries and Forestry, Canberra (Australia), 190 pp.
- Hilborn, R., K. Stokes, J.J. Maguire, T. Smith, L.W. Botsford, et al., 2004: When can marine reserves improve fisheries management? Ocean & Coastal Management, 47(3–4), 197–205.
- Holmes, B., 1997: Destruction follows in trawlers’ wake. New Scientist, 14 June.
- Holt, S., 2004: Sharing our seas with whales and dolphins. FINS- Newsletter of ACCOBAMS, 1(1), 2–4.
- Hutchings, J.A., 2000: Collapse and recovery of marine fishes. Nature, 406(6798), 882–885.
- Hutchings, J.A., 2004: Evolutionary biology—The cod that got away. Nature, 428(6986), 899–900.
- Hutchings, J.A. and M. Ferguson, 2000: Temporal changes in harvesting dynamics of Canadian inshore fisheries for northern Atlantic cod, Gadus morhua. Canadian Journal of Fisheries and Aquatic Sciences, 57(4), 805–814.
- ICES (International Council for the Exploration of the Sea), 2003: Seamounts— Hotspots of Marine Life. [online] Cited November 2004. Available at http:// www.ices.dk/marineworld/seamounts.asp.
- Idyll, C.P., 1978: The Sea Against Hunger—Harvesting the Oceans to Feed a Hungry World. Thomas Y. Crowell Company, New York, NY (USA), 222 pp. Instituto de Mar del Peru, 2004: II Panel Internacional de expertos para la evaluacion de la merluz peruana [online] Cited November 2004. Available at http://www.imarpe.gob.pe/imarpe/panel_2_merlu_03marzo_2004.php
- IPCC (Intergovernmental Panel on Climate Change), 2003: Climate Change 2001: The Scientific Basis. Contribution of Working Group I to the Third Assessment Report. J.T. Houghton, Y. Ding, D.J. Griggs, M. Noguer, P.J. van der Linden, X. Dai, K. Maskell, C.A. Johnson (eds.). Cambridge University Press, Cambridge (UK), 892 pp.
- Jackson, J.B.C., M.X. Kirby, W.H. Berger, K.A. Bjorndal, L.W. Botsford, et al., 2001: Historical overfishing and the recent collapse of coastal ecosystems. Science, 293(5530), 629–638.
- Jameson, S.C., M.V. Erdmann, G.R. Gibson Jr., and K.W. Potts, 1998: Development of Biological Criteria for Coral Reef Ecosystem Assessment. Atoll Research Bulletin No.450, United States Environmental Protection Agency, Office of Science and Technology, Health and Ecological Criteria Division; Smithsonian Institution, Washington, D.C. (USA), 102 pp.
- Japan Times, 2001: One tuna fetches 20 million yen at Tsukiji. January 6.
- Jennings, S., J.K. Pinnegar, N.V.C. Polunin, and K.J. Warr, 2001: Impacts of trawling disturbance on the trophic structure of benthic invertebrate communities. Marine Ecology-Progress Series, 213, 127–142.
- Johannes, R.E., 1981: Words of the Lagoon: Fishing and Marine Lore in the Palau District of Micronesia. University of California Press, Berkley (USA), 245 pp.
- Johannes, R.E., M.M.R. Freeman, and R.J. Hamilton, 2000: Ignore Fishers’ Knowledge and Miss the Boat. Fish and Fisheries, 1, 257–271.
- Jones, I.S.F., 2001: The global impact of ocean nourishment. Paper presented at the First National Conference on Carbon Sequestration. NETL Publications, Washington, D.C. (USA).
- Kaczynski, V.M. and D.L. Fluharty, 2002: European policies in West Africa: who benefits from fisheries agreements? Marine Policy, 26(2), 75–93.
- Kaschner, K., 2004: Modelling and mapping of resource overlap between marine mammals and fisheries on a global scale. Zoology, Ph.D., University of British Columbia (UBC), Vancouver (Canada).
- Kaschner, K. and D. Pauly, 2004: Competition between marine mammals and fisheries: food for thought. Report for the Humane Society by the Fisheries Centre, UBC, Vancouver (Canada), 28 pp.
- Kelleher, K., 2004: Discards on the world’s marine fisheries: An update. FAO Fisheries Technical Paper No. 470, FAO, Rome (Italy), 131 pp. Kock, K.H., 1991: The state of exploited fish stocks in the Southern Ocean—a review. Archiv fu¨r Fischereiwissenschaft, 41(1), 1–66.
- Kock, K.H., 1992: Antarctic Fish and Fisheries. Cambridge University Press, Cambridge (UK), 359 pp.
- Kock, K.H., 2001: The direct influence of fishing and fishery-related activities on non-target species in the Southern Ocean with particular emphasis on longline fishing and its impact on albatrosses and petrels—a review. Reviews in Fish Biology and Fisheries, 11(1), 31–56.
- Koslow, J.A., G.W. Boehlert, J.D.M. Gordon, R.L. Haedrich, P. Lorance, and N. Parin, 2000: Continental slope and deep-sea fisheries: implications for a fragile ecosystem. Ices Journal of Marine Science, 57(3), 548–557.
- Kulka, D.W., J.S. Wroblewski, and S. Narayanan, 1995: Recent Changes in the Winter Distribution and Movements of Northern Atlantic Cod (Gadus- Morhua Linnaeus, 1758) on the Newfoundland-Labrador Shelf. Ices Journal of Marine Science, 52(6), 889–902.
- Kurien, J., 1998: Does international trade in fishery products contribute to food security? [online] Proceedings of the FAO E-mail conference on Fish Trade and Food Security 19 October—12 December 1998. Food and Agriculture Organization of the United Nations, Fish Utilization and Marketing Service. Available at http://www.tradefoodfish.org/articles.php?pageid art&article article01.
- Kurien, J., 2004: Responsible Fish Trade and Food Security: Toward understanding the relationship between international fish trade and food security. Conducted jointly by the Food and Agriculture Organisation of the United Nations (FAO) and the Royal Norwegian Ministry of Foreign Affairs, Rome (Italy), 121 pp. Labropoulou, M., A. Machias, and N. Tsimenides, 1999: Habitat selection and diet of juvenile red porgy, Pagrus pagrus (Linnaeus, 1758). Fishery Bulletin, 97(3), 495–507.
- Lavigne, D.M., 2002: Harp seal—Pagophilus groenlandicus. In: Encyclopedia of Marine Mammals, W.F. Perrin, B. Wu¨rsig, and J.G.M. Thewissen (eds.), Academic Press, San Diego, CA (USA), 560–562.
- Lilly, G.R., D.G. Parsons, and D.W. Kulka, 2000: Was the increase in shrimp biomass on the northeast Newfoundland shelf a consequence of a release in predation pressure from cod? Journal of Northwest Atlantic Fisheries Science, 27, 45–61.
- Lleonart, J., 1999: Precautionary approach and Mediterranean fisheries. Paper presented at the Precautionary approaches to local fisheries and species introductions in the Mediterranean. CIESM Workshop Series 7, 23–26 september, Kerkennah (Tunisia), 15–24 pp.
- Lloret, J., J. Lleonart, I. Sole, and J.M. Fromentin, 2001: Fluctuations of landings and environmental conditions in the north-western Mediterranean Sea. Fisheries Oceanography, 10(1), 33–50.
- Longhurst, A. and D. Pauly, 1987: Ecology of Tropical Oceans. Academic Press, San Diego, CA (USA), 407 pp.
- Longhurst, A., S. Sathyendranath, T. Platt, and C. Caverhill, 1995: An Estimate of Global Primary Production in the Ocean from Satellite Radiometer Data. Journal of Plankton Research, 17(6), 1245–1271.
- Longhurst, A.R., 1998: Ecological Geography of the Sea. Academic Press, San Diego, CA (USA), 398 pp.
- Mace, P.M., 1997: Developing and Sustaining World Fisheries Resources: The State of the Science and Management. In: Proceedings of the 2nd World Fisheries Congress: Developing and Sustaining World Fisheries Resources—The State of Science and Management:, D.A. Hancock, D.C. Smith, A. Grant, and J.P. Beumer (eds.), CSIRO Publishing, Collingwood (Australia), 1–20.
- Mace, P.M., 2001: A new role for MSY in single-species and ecosystem approaches to fisheries stock assessment and management. Fish and Fisheries, 2(1), 2–32.
- Macinko, S. and D.W. Bromley, 2002: Who Owns America’s Fisheries. Island Press Publication Services, Washington, D.C. (USA), 48 pp.
- MacPherson, E., 2002: Large-scale species-richness gradients in the Atlantic Ocean. Proceedings of the Royal Society of London Series B-Biological Sciences, 269(1501), 1715–1720.
- Malakoff, D., 1998: Fisheries science—Papers posit grave impact of trawling. Science, 282(5397), 2168–2169.
- McGinn, A.P., 1999: Worldwatch Paper No. 145: Safeguarding the Health of Oceans. Worldwatch Institute, Washington, D.C. (USA), 87 pp.
- McLean, R.F. and A. Tsyban, 2001: Coastal Zones and Marine Ecosystems. In: Climate Change 2001: Working group II: Impacts, Adaptation and Vulnerability, J.J. McCarthy, O.F. Canziani, N.A. Leary, D.J. Dokken, and K.S. White 510 Ecosystems and Human Well-being: Current State and Trends (eds.), Published for the Intergovernmental Panel on Climate Change, Cambridge University Press, Cambridge (UK), 345–382.
- McNutt, M.K., 2002: Developing the Ocean Opportunities and Responsibilities: A new era of ocean exploration will feature intelligent vehicles that track fish populations and ‘‘drifters’’ that constantly monitor ocean temperature and current. The Futurist, Jan–Feb, 38–43.
- Milazzo, M.J., 1998: Subsidies in World Fisheries. A Reexamination. WorldBank Technical Paper No. 406, The WorldBank,Washington, D.C. (USA), 86 pp.
- Mishra, B.K., 1997: Fisheries Co-operatives in India. Co-op Dialogue, 7(2), 28–31.
- Morales, H.L., 1993: Deep Sea, Long Hours: The Condition of Fishworkers on Distant Water Vessels. Samudra, 7, 11.
- Munro, G.R. and U.R. Sumaila, 2002: The impact of subsidies upon fisheries management and sustainability: the case of the North Atlantic. Fish and Fisheries, 3(4), 233–250.
- Myers, R.A. and B. Worm, 2003: Rapid worldwide depletion of predatory fish communities. Nature, 423(6937), 280–283. NEFSC (Northeast Fisheries Science Center), 2002: Assessment of 20 Northeast Groundfish Stocks through 2001. A Report of the Groundfish Assessment Review Meeting (GARM), Northeast Fisheries Science Center, Woods Hole, Massachusetts, October 8–11, 2002. Northeast Fisheries Science Center Reference Document 2–16, Woods Hole, MA (USA), 544 pp.
- Neis, B., R. Jones, and R. Ommer, 2000: Food Security, Food Self-Sufficiency, and Ethical Fisheries Management. In: Just Fish: Ethics and Canadian Marine Fisheries, H. Coward, R. Ommer, and T. Pitcher (eds.). Social and Economic Papers 23, Institute of Social and Economic Research, Memorial University of Newfoundland, St John’s (Canada), 154–173.
- NMFS (National Marine Fisheries Service), 2004: NMFS Strategic Plan for Fisheries Research. Technical Memorandum NMFS F/SPO-61, U.S. Department of Commerce, National Oceanic and Atmospheric Administration, Silver Spring, MD (USA), 148 pp.
- NRC (National Research Council), 2001: Marine Protected Areas: Tools for Sustaining Ocean Ecosystem. National Academy Press, Washington, D.C. (USA), 288 pp.
- OECD (Organisation for Economic Co-operation and Development), 2000: Transition to Responsible Fisheries Economic and Policy Implications. Paris (France), 268 pp.
- Okey, T.A., 2003: Membership of the eight Regional Fishery Management Councils in the United States: are special interests over-represented? Marine Policy, 27(3), 193–206.
- Panayotou, T., 1982: Management concepts for small-scale fisheries: Economic and social aspects. FAO Fisheries Technical Paper 228, Fisheries and Agriculture Organization of the United Nations (FAO), Rome (Italy), 53 pp.
- Pandolfi, J.M., R.H. Bradbury, E. Sala, T.P. Hughes, K.A. Bjorndal, et al. 2003: Global trajectories of the long-term decline of coral reef ecosystems. Science, 301(5635), 955–958.
- PISCO (Partnership for Interdisciplinary Studies of Coastal Oceans), 2002: The Science of Marine Reserves. Cited December 2004. Available at http: //www.piscoweb.org
- Pauly, D., 1997: Small-scale fisheries in the tropics: marginality, marginalization, and some implications for fisheries management. In: Proceedings of the 20th American Fisheries Society Symposium: Global Trends -Fisheries Management, 14–16 June 1994, Seattle, WA (USA), E.K. Pikitich, D.D. Huppert, and M. Sissenwine (eds.), American Fisheries Society, Bethesda, MD (USA), 40–49.
- Pauly, D., V. Christensen, J. Dalsgaard, R. Froese, and F. Torres Jr., 1998: Fishing down marine food webs. Science, 279, 860–863.
- Pauly, D., J. Alder, E. Bennett, V. Christensen, P. Tyedmers, and R. Watson, 2003: The future for fisheries. Science, 302(5649), 1359–1361.
- Pauly, D., V. Christensen, S. Gue´nette, T.J. Pitcher, U.R. Sumaila, C.J. Walters, R. Watson, and D. Zeller, 2002: Towards sustainability in world fisheries. Nature, 418(6898), 689–695.
- Petersen, E., 2003: The catch in trading fishing access for foreign aid. Marine Policy, 27(3), 219–228.
- Phyne, J. and J. Mansilla, 2003: Forging linkages in the commodity chain: The case of the Chilean salmon farming industry, 1987–2001. Sociologia Ruralis, 43(2), 108– .
- Pike, S.T. and A.F. Spilhaus, 1962: Marine Resources. National Academy of Sciences, National Research Council, Washington, D.C.
- Portner, H.O., B. Berdal, R. Blust, O. Brix, A. Colosimo, et al., 2001: Climate induced temperature effects on growth performance, fecundity and recruitment in marine fish: developing a hypothesis for cause and effect relationships in Atlantic cod (Gadus morhua) and common eelpout (Zoarces viviparus). Continental Shelf Research, 21(18–19), 1975–1997.
- Preston, G.L., 1997: Rerview of Fishery Management Issues and Regimes in the Pacific Island Region. South Pacific Regional Environment Programme, South Pacific Commission, South Pacific Forum Agency, Noumea (New Caledonia), 72 pp.
- Price, A.R.G., 2002: Simultaneous ‘hotspots’ and ‘coldspots’ of marine biodiversity and implications for global conservation. Marine Ecology Progress Series, 241, 23–27.
- Richardson, A. J. and D. S. Schoeman, 2004: Climate impact on plankton ecosystems in the Northeast Atlantic. Science 305:1609–1612.
- Roberts, C.M. and J.P. Hawkins, 1999: Extinction risk in the sea. Trends in Ecology & Evolution, 14(6), 241–246.
- Roberts, C. and J. P. Hawkins. 2000: Fully-protected marine reserves: a guide. WWD Endangered Seas Campaign, WWF, Washington D. C. (USA) and University of York, York (UK), 131 pp.
- Roberts, C.M., C.J. McClean, J.E.N. Veron, J.P. Hawkins, G.R. Allen, et al., 2002: Marine biodiversity hotspots and conservation priorities for tropical reefs. Science, 295(5558), 1280–1284.
- Rolden, R. and R. Sievert, 1993: Coasta Resources Management: A Maunual for Government Officials and Community Organizers, Department of Agriculture, Manilla (Philippines)
- Rudd, M.A. and M.H. Tupper, 2002: The impact of Nassau grouper size and abundance on scuba diver site selection and MPA economics. Coastal Management, 30(2), 133–151.
- Sadovy, Y., 2001: The threat of fishing to highly fecund fishes. Journal of Fish Biology, 59, 90–108.
- Sadovy, Y. and W.L. Cheung, 2003: Near extinction of a highly fecund fish: The one that nearly got away. Fish and Fisheries, 4(1), 86–99.
- Sadovy, Y.J. and A.C.J. Vincent, 2002: Ecological Issues and the Trades in Live Reef Fishes. In: Coral reef fishes: Dynamics and diversity in a complex ecosystem, P.F. Sale (ed.), Academic Press, San Diego, CA (USA), 391–420.
- Sadovy, Y., M. Kulbicki, P. Labrosse, Y. Letourneur, P. Lokani, and T.J. Donaldson, 2003a: The humphead wrasse, Cheilinus undulatus: synopsis of a threatened and poorly known giant coral reef. Reviews in Fish Biology and Fisheries, 13(3), 327–364.
- Sadovy, Y.J., T.J. Donaldson, T.R. Graham, F.McGilvray, G.J. Muldoon, M.J. Phillips, M.A. Rimmer, A. Smith, and B. Yeeting, 2003b: While Stocks Last: The Live Reef Food Fish Trade. Asian Development Bank (ADB), Manila (Philippines), 169 pp.
- Sardjono, I., 1980: Trawlers banned in Indonesia. ICLARM Newsletter, 3(4), 3.
- SAUP (Sea Around Us Project), 2005: Sea Around Us database. [online] Cited 5 February. Available at http://www.searoundus.org.
- Schwartzlose, R.A., J. Alheit, A. Bakun, T.R. Baumgartner, R. Cloete, et al., 1999:Worldwide large-scale fluctuations of sardine and anchovy populations. South African Journal of Marine Science, 21, 289–347.
- Sherman, K. and A.M. Duda, 1999: Large marine ecosystems: An emerging paradigm for fishery sustainability. Fisheries, 24(12), 15–26.
- Sissenwine, M.P. and P.M. Mace, 2003: Governance for responsible fisheries: an ecosystem approach. In: Responsible fisheries in the marine ecosystem, M. Sinclair and G. Valdimarsson (eds.), Fisheries and Agriculture Organization of the United nations (FAO), Rome (Italy) and CAB International, Wallingford (UK), 363–380.
- Smith, M. and C. Taal, 2001: EU Market Survey 2001: Fishery Products. Centre for the Promotion of Imports from Developing Countries (CPI), Rotterdam (Netherlands), 108 pp.
- South Pacific Regional Environment Program, 2003: Pacific Islands Regional Ocean Policy. [online] Cited November 2004. Available at http:// www.spc.int/piocean/policy/oceanpolicy.htm.
- Sprague, L.M. and J.H. Arnold, 1972: Trends in the use and prospects for the future harvest of world fisheries resources. J. Amer. Oil. Chemist. Soc., 49: 345–350
- St Clair, J., 1997: Fishy Business. In These Times, May 26, 14–16.
- Stehn, R.A., K.S. Rivera, S. Fitzgerald, and K.D. Whol, 2001: Incidental catch of Seabirds by Longline Fisheries in Alaska. In: Seabird Bycatch: Trends, Roadblocks and Solutions, E.F. Melvin and J.K. Parrish (eds.), Annual Meeting of the Pacific Seabird Group, February 26–27, 1999, Blaine Washington. University of Alaska Sea Grant, AK-SG-01–01, Fairbanks, AK (USA), 204.
- Stephens, P. A., and W. J. Sutherland, 1999: Consequences of the Allee effect for behaviour, ecology and conservation. Trends in Ecology and Evolution 14: 401–405.
- Stergiou, K.I., E.D. Christou, D. Georgopoulos, A. Zenetos, and C. Souvermezoglou, 1997: The Hellenic Seas: physics, chemistry, biology and fisheries. Oceanography Marine Biology: an Annual Review, 35(415–538). Marine Fisheries Systems 511
- Stewart, B.S., P.K. Yochem, H.R. Huber, R.L. DeLong, R.J. Jameson, et al., 1994: History and present status of the northern elephant seal population. In: Elephant seals: Population ecology, behavior and physiology, B.J. LeBoeuf and R.M. Laws (eds.), University of California Press, Berkeley, CA (USA), 29–48.
- Sumaila, R.U., 2001: Generational Cost Benefit Analysis for Evaluating Marine Ecosystem Restoration. In: Fisheries Impacts on North Atlantic Ecosystems: Evaluations and Policy Exploration, T.J. Pitcher, U.R. Sumaila, and D. Pauly (eds.), Fisheries Centre Research Reports 9(5) 3–9.
- Sumaila, U.R. and C. Walters, 2005: Intergenerational discounting: a new intuitive approach. Ecological Economics, 52, 135–142.
- Taylor, B.L., 1997: Defining ‘‘population’’ to meet management objectives for marine mammals. In: Molecular Genetics of Marine Mammals., A.E. Dizon and a.W.F.P. S. J. Chivers (eds.). Society for Marine Mammalogy Special Publication 3, The Society for Marine Mammalogy, Lawrence, KA (USA), 49–65.
- Taylor, B.L. and A.E. Dizon, 1999: First policy then science: why a management unit based solely on genetic criteria cannot work. Molecular Ecology, 8(12), S11–S16.
- Thiel, H. and J.A. Koslow (eds.), 2001: Managing Risks to Biodiversity and the Environment on the High Sea, Including Tools Such as Marine Protected Areas— Scientific Requirements and Legal Aspects. Proceedings of the Expert Workshop held at the International Academy for Nature Conservation Isle of Vilm (Germany), 27 February—4 March. German Federal Agency for Nature Conservation, Bonn (Germany), 214 pp.
- Tomey, W.A., 1997: Review of Developments in the World Ornamental Fish Trade: Update, Trends and Future Prospects, in K.P.P. Namibar and T. Singh (eds.), Sustainable Aquaculture: Proceedings of the INFOFISH-AQUATECH ’96 International Conference on Aquaculture, Kuala Lumpur, Malaysia.
- Tomlinson, Z., 2003: Abrogation or regulation? How Anderson v. Evans discards the Makah’s treaty whaling right in the name of conservation necessity. Washington Law Review 78 (4): 1101–1129
- Tserpes G. and P. Peristeraki, 2002: Trends in the abundance of demersal species in the southern Aegean Sea.Scientia Marina, 66 (Suppl 2), 243–252.
- Tsukayama, I. and M.L.D. Palomares, 1987: Monthly catch and catch composition of Peruvian anchoveta (Engraulis ringens) (Northern-Central stock, 4–14 S), 1953 to 1982. In: The Peruvian anchoveta and its upwelling ecosystem: three decades of change, D. Pauly and I. Tsukayama (eds.), ICLARM, Manila (Philippines), ICLARM Stud. Rev. 15. 89–108.
- UN (United Nations), 1995: Agreement for the Implementation of the Provisions of the United Nations Convention on the Law of the Sea of 10 December 1982 Relating to the Conservation and Management of Straddling Fish Stocks and Highly Migratory Fish Stocks. United Nations Conference on Straddling Fish Stocks and Highly Migratory Fish Stocks. [online] Cited No- vember 2004. Available at http://www.un.org/Depts/los/fish_stocks_con ference/fish_stocks_conference.htm.
- UNEP (United Nations Environment Programme), 2002b: Environmental impact of trade liberalization and trade-linked measures in the fisheries sector. National Oceanographic and Fisheries Research Centre, Nouadhibou (Mauritania).
- UNEP, 2002a: Integrated Assessment of Trade Liberalization and Trade-Related Policies: A Country Study on the Fisheries Sector in Senegal. United Nations Environment Programme, 81 pp.
- Walters, C. and J. J. Maguire, 1996: Lessons for stock assessment from the northern cod collapse. Reviews in Fish Biology and Fisheries, 6(2), 125–137.
- Warwick, R.M. and K.R. Clarke, 2001: Practical measures of marine biodiversity based on relatedness of species. In: Oceanography and Marine Biology, Vol 39. 39, 207–231.
- Warwick, R.M. and S.M. Turk, 2002: Predicting climate change, effects on marine biodiversity: comparison of recent and fossil molluscan death assemblages. Journal of the Marine Biological Association of the United Kingdom, 82(5), 847–850.
- Watson, R. and D. Pauly, 2001: Systematic distortions in world fisheries catch trends. Nature, 414(6863), 534–536.
- Watson, R., D. Pauly, V. Christensen, R. Froese, A. Longhurst, et al., 2003: Mapping natural ocean regions and LMEs. In: Large Marine Ecosystems of the World: Trends in Exploitation, Protection, and Research, G. Hempel and K. Sherman (eds.), Elsevier, New York, NY (USA), 375–397.
- Welch, D.W., Y. Ishida, and K. Nagasawa, 1998: Thermal limits and ocean migrations of sockeye salmon (Oncorhynchus nerka): long-term consequences of global warming. Canadian Journal of Fisheries and Aquatic Sciences, 55(937– 948).
- Wilde, G.R., K.L. Pope, and R.E. Strauss, 2003: Estimation of fishing tournament mortality and its sampling variance. North American Journal of Fisheries Management, 23(3), 779–786.
- Willis, T.J., R.B. Millar, R.C. Babcock, and N. Tolimieri, 2003: Burdens of evidence and the benefits of marine reserves: putting Descartes before des horse? Environmental Conservation, 30(2), 97–103.
- Wiltshire, J., 2001: Future Prospects for the Marine Minerals Industry. Under- Water Magazine, May/June.
- Worm, B., H.K. Lotze, and R.A. Myers, 2003: Predator diversity hotspots in the blue ocean. Proceedings of the National Academy of Sciences of the United States of America, 100(17), 9884–9888.
- WWF (World Wide Fund for Nature), 2002: Koster/Yttre Hvaler—A Potential MPA. [online] WWF Germany. Cited November 2004. Available at http:// www.ngo.grida.no/wwfneap/Publication/briefings/Hvaler.pdf.
- WWF/IUCN (World Conservation Union), 2001: The status of natural resources on the high-seas. Gland (Switzerland).
Terms of Use
The copyright for material on this page is the property of the World Resources Institute. Click here for the Terms of Use (Ecosystems and Human Well-Being: Volume 1: Current State and Trends: Marine Systems).
Disclaimer: This chapter is taken wholly from, or contains information that was originally written for the Millennium Ecosystem Assessment as published by the World Resources Institute. The content has not been modified by the Encyclopedia of Earth.
|
|