Biofuels: A Greenhouse-Gas Free Energy Source?
Most modern engines have the flexibility to combust mixtures containing biofuels. Biofuels are those produced from living materials and include bioethanol, biomethane, and biodiesel. The energy in biofuels originally derives from photosynthesis. Bioethanol is produced through the fermentation of a carbohydrate such as glucose (C6H12O6) into Ethanol, and combustion of ethanol yields chemical energy. Summing these reactions together yields the simple energy conversion:
light (solar electromagnetic energy) --> chemical energy
In theory, the chemical energy in bioethanol propels a vehicle without any net production of greenhouse gases (Greenhouse Gases)..
Biomethane or biogas follows a similar series of reactions. Carbohydrates produced via photosynthesis are converted into methane (CH4), the main component of natural gas, and combustion of this “natural” natural gas (as opposed to natural gas extracted as a fossil fuel) releases carbon dioxide (CO2) in amounts equal to those recently removed from the atmosphere during photosynthesis. Once again, a vehicle propelled by biomethane, at least in theory, does not contribute additional greenhouse gases to the atmosphere.
Biodiesel involves a more complex series of chemical reactions. In brief, several biochemical pathways synthesize fats (a class of biochemicals composed of triglycerides) from photosynthetic carbohydrates. [1] Plants store some of these fats as “vegetable oils” in seeds to serve as an energy source for germinating embryos. Extracting these oils and treating them with methanol (CH2OH) or sometimes ethanol (C2H5OH) in the presence of a strong base (e.g., NaOH) produces methyl or ethyl esters of fatty acids. Methyl esters and ethyl esters of fatty acids have combustion properties very similar to those of petrodiesel. Combustion of biodiesel, however, returns to the atmosphere (Earth's atmosphere) CO2 that was recently withdrawn during photosynthesis, whereas combustion of petrodiesel returns to the atmosphere CO2 that was withdrawn millennia ago.
The reactions to produce biodiesel also generate glycerol, a substance used in pharmaceuticals (e.g., cough syrup), personal care products (e.g., soap), and foods (e.g., a solvent for food colorings and vanilla flavoring). Expanded production of biodiesel has created a glut of glycerol on the world market, and its value has plunged. New commercial applications of glycerol, such as its conversion into propylene glycol or ethanol, are still under development. Biofuels, in practice, are not even close to being neutral with respect to generation of greenhouse gases because their production and use deviate significantly from 100% efficiency. Inefficiencies arise during production of biomass, biologically generated material; transport of biomass to a processing facility; conversion of biomass to biofuels; distribution of biofuels; and combustion of biofuels in vehicles.
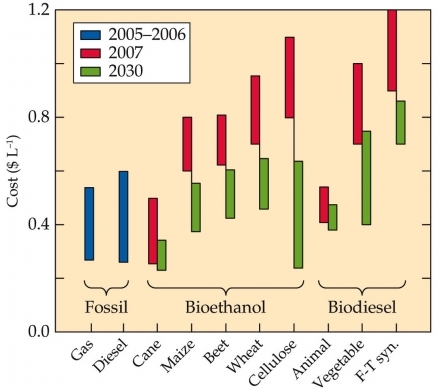
Fundamental questions remain about the extent to which biofuels could supplant fossil fuels. Different budget analyses for biofuels reach different conclusions. Estimates of greenhouse gas emissions per unit of energy obtained from biofuels vary tenfold. In addition, some analyses indicate that the energy in biofuels is nearly double that of the fossil fuels expended to grow the biomass and process it, whereas a few others find that the energy in biofuels does not even match that of the fossil fuels expended to produce it.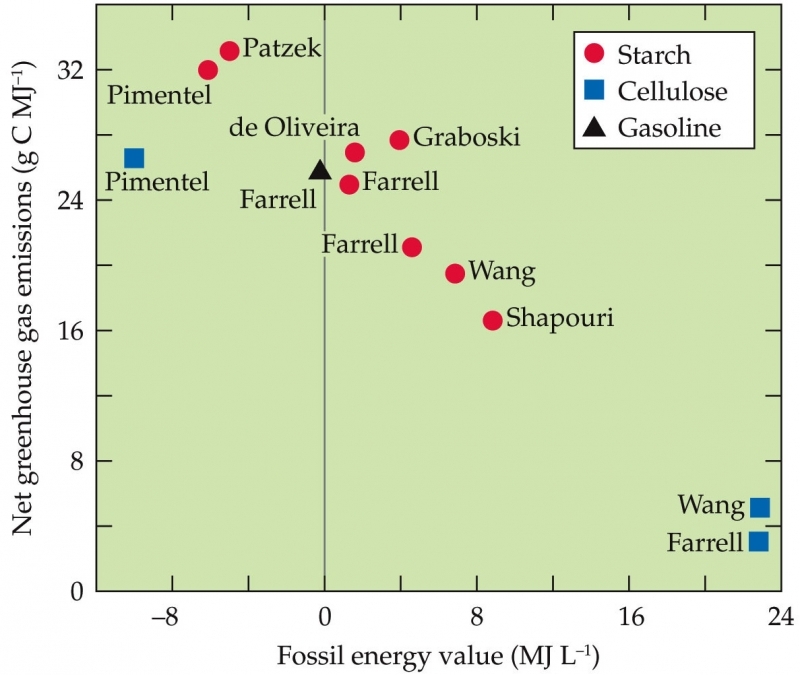
Net greenhouse gas emissions (gram carbon equivalents per 106 joules energy in the ethanol) during the growth and processing of bioethanol from maize starch or switchgrass cellulose as a function of fossil energy value.
Several assumptions are responsible for these discrepancies. Some analyses assume that sustainable production of biomass will require substantial fertilization and irrigation, together with prolonged fallow periods, whereas others assume that cellulosic crops will require minimal additions of fertilizer and water. Analyses also differ in their assumptions on the proportion of energy for processing that might derive from the biomass itself. Some argue that as much biomass (such as sugarcane stubble) as possible should remain in the field to maintain soil fertility; others argue that leaving biomass in the field just lets a valuable resource rot. Some predict that energy-rich lignin, when separated from biomass, can provide much of the energy for processing; others point out that current methods for removing lignin from biomass expend large amounts of fossil fuel energy.
As the biofuels industry matures, many of these discrepancies will be resolved, but how realistic is the goal of replacing 30% of oil use in the United States with alternative fuels by 2030? To replace 30% of the U.S. oil consumption with bioethanol from maize grain would require cultivation of 808 million hectares of maize per year just for this purpose. For perspective, the total land area of the United States, including Alaska and Hawaii, is 916 million hectares, and 38 million hectares are currently in maize production.
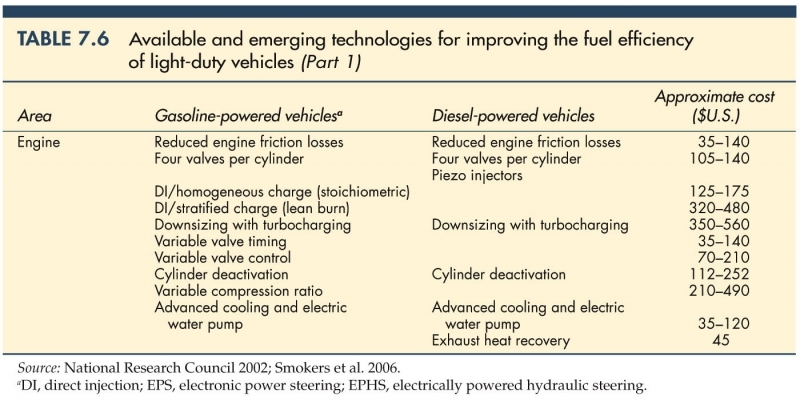
Table showing available and emerging technologies for improving fuel efficiency.
Even optimistic estimates for cellulosic ethanol indicate that biomass to meet the “30-by-30” goal would cover 117 million hectares, an area larger than the states of Texas and California combined. We can only hope that there is a calculation error somewhere and that the “30-by-30” goal at least has some possibility of success.
[1] Taiz, L. and E. Zeiger (2006) Plant Physiology, 4th Edition. Sinauer Associates, Sunderland, MA.
This is an excerpt from the book Global Climate Change: Convergence of Disciplines by Dr. Arnold J. Bloom and taken from UCVerse of the University of California.
©2010 Sinauer Associates and UC Regents