The Makeup of Earth's Atmosphere
Energy and Electromagnetic Radiation
Words seem to fail certain concepts from physics and chemistry. These concepts, however, fit into a consistent mathematical framework and provide the core equations of models that both accurately reconstruct Earth’s climate history and successfully predict tomorrow’s weather. Energy is one of these concepts.
Energy, according to a classical definition, is the ability to do work, where work is a force applied over a distance, and a force is that which has the ability to change the motion of a particle. Alternatively, energy is the capacity to change environmental conditions; for example, the capacity to change the temperature of a substance. The conservation of energy law, or the first law of thermodynamics, states that this capacity, this energy, is never created nor destroyed; it is just transformed from one form into another.
Nearly all of the energy impinging on Earth is electromagnetic radiation. The interaction between electromagnetic radiation and gases in Earth’s atmosphere is the basis of the so-called greenhouse effect. The conservation of energy law dictates that the amount of electromagnetic radiation entering Earth’s sphere of influence must equal the amount leaving the sphere.
Most of the gases in Earth’s atmosphere are inert. The bulk (78%) is dinitrogen (N2), and substantial portions are the noble gases argon (0.93%), neon (0.0018%), helium (0.00052%), krypton (0.00011%), and xenon (0.0000009%). These gases remain prevalent in the atmosphere because they neither engage in chemical reactions with other elements, nor do they interact with solar electromagnetic radiation; that is, they are totally transparent. Of the remaining gases,
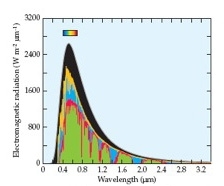
• Dioxygen (O2), which constitutes 21% of the atmosphere, absorbs in the visible and near infrared (Figure 3.34),
• Water vapor (H2O), which composes anywhere from 1% to 4%, absorbs at a number of wavelengths in the visible, near infrared, and mid-infrared,
• Ozone (O3), which is present at up to 0.0000007%, absorbs broadly throughout the ultraviolet, visible, near infrared, and mid infrared wavelengths, and
• Carbon dioxide (CO2; 0.038%), methane (CH4; 0.00018%), and nitrous oxide (N2O; 0.000032%) transmit visible wavelengths and absorb at various wavelengths in the near and mid infrared.
Absorption by all of these gases account for the notches in the solar radiation reaching Earth’s surface. Earth acts approximately like a blackbody with an effective temperature of about 287 K (14°C) and thereby emits a maximum amount of energy at 10.1 µm, a wavelength that falls in mid-infrared. Several gases in the atmosphere absorb some of the wavelengths that Earth emits. In particular, CO2, CH4, and N2O are considered greenhouse gases because they transmit the strongest wavelengths from the sun (0.3 µm to 1 µm), but they absorb major wavelengths emitted from Earth (2 µm to 8 µm). When gas molecules absorb infrared radiation, their vibrational energy increases, and so does their temperature.
Warmer gas molecules emit electromagnetic radiation at shorter, more energetic wavelengths than do cooler gas molecules. In accordance with the conservation of energy law (which states that energy of the incident electromagnetic radiation equals the energy of the emitted electromagnetic radiation plus the kinetic energy in the motion of the gas molecules), the wavelengths emitted by the warmer gas molecules are longer and less energetic than those originally absorbed. These gas molecules, however, move more rapidly after absorbing electromagnetic radiation and collide with other gas molecules that, in turn, move more rapidly and emit additional electromagnetic radiation.
Putting this all together, a volume of gas molecules emits electromagnetic radiation proportional to its temperature. When illuminated with electromagnetic radiation of appropriate wavelengths, some of the molecules in the volume will absorb this energy and increase their movements. Through collisions of these molecules with their neighbors (molecular “moshing”), movement of the volume as a whole increases. The gas volume, therefore, will increase in temperature and emit electromagnetic radiation at wavelengths that are shorter than the gas volume emitted before illumination, but longer than the incident radiation. Energy is conserved because the energy in the shorter wavelength electromagnetic radiation absorbed by a few molecules equals the total energy in the longer wavelength radiation emitted by many molecules.
This is an excerpt from the book Global Climate Change: Convergence of Disciplines by Dr. Arnold J. Bloom and taken from UCVerse of the University of California.
©2010 Sinauer Associates and UC Regents