Technological change in the sulfur industry
Contents
Production history
Sulfur, which also is known as brimstone (“the stone that burns”), has been used in small quantities for thousands of years. Early humans used sulfur as a colorant for cave drawings, as a fumigant, in medicine, and as incense.
By 2000 B.C., the Egyptians began using sulfur in the bleaching of linen textiles. Homer referred to its use as a fumigant in "The Odyssey". In the 5th century B.C., during the Peloponnesian War, the Greeks used burning sulfur and pitch to produce suffocating gases. The Romans combined brimstone with pitch, tar, and other combustible materials to produce the first incendiary weapons. Through the use of alchemy during the Golden Age of Arabic Science about A.D. 700, Arabs were probably the first to produce sulfuric acid.
Sulfur is a necessary ingredient in gunpowder, which was developed in China in the 10th century. Gunpowder’s introduction into Europe led to its use in warfare in the 14th century and that made sulfur an important mineral commodity for the first time.However, not until the birth of the science of chemistry in the 1700s and the growth of chemical industries in the 1800s did sulfur become of major significance to the world.Early chemists soon recognized the importance of sulfuric acid as the cheapest and most versatile of mineral acids, and it rapidly became one of the most common acids in the chemical industry.
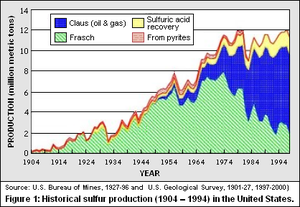
As shown in Figure 1, the United States had an annual sulfur production in 1930 of about 1.3 million metric tons (Mt). By 1963, the production of sulfur had almost quintupled. During that same period, the worldwide production of sulfur had quadrupled. This growth resulted from accelerated demand and production of phosphate fertilizers, which used about 60 to 70 percent of the sulfur production, and large quantities of sulfur produced as a byproduct from newly discovered Canadian natural gas.
Between 1984 and 1996, sulfur production in some countries shifted from discretionary to mandated sources, and world production outpaced increasing demand. Overall, between 1920 and 1998, the constant-dollar (1998) unit value for sulfur was decreasing.
The main uses for sulfur in 1999 were phosphate fertilizer production, 69 percent; catalysis in petroleum refinery alkylation units, 16 percent; metals industry processes (leaching and pickling), 7 percent; chemicals, 4 percent; and other uses, 4 percent.
Technology
Claus process sulfur recovery from natural gas and crude oil
The Frasch process was developed to take advantage of a very special set of geological circumstances involving large sulfur deposits in the Gulf of Mexico and along the Gulf Coast area. The use of the Frasch process grew steadily from 1904 to about 1970 (see Figure 1). It was the principal source of U.S. sulfur until 1982 when the Claus processfor recovering elemental sulfur from the processing of natural gas (Natural gas processing) and petroleum crude oil began to make a substantial inroad to the sulfur market..The Claus process was first used to recover sulfur from crude oil and and natural gas in about 1950. Raw natural gas usually contains significant amount of hydrogen sulfide which is very odorous and toxic. Petroleum refineries produce by-product fuel gas (consumed within the refineries) as well as gasoline and other end-use products. The by-product fuel gas and most of the end-use products contain various sulfur compounds all of which are removed in the form of gaseous hydrogen sulfide during the refining of the crude oil. The gaseous hydrogen sulfide is then catalytically converted to elemental sulfur in Claus process units.From 1950 to 1975, the amount of sulfur recovered from oil refining grew steadily. Since 1975, higher growth rates for sulfur recovery from petroleum refining can be attributed to two factors. First, sulfur dioxide emissions (Emissions factors) limits mandated in the Clean Air Act of 1970 drove producers to strip the sulfur from fuel before combustion. In 1997, sulfur from mandated sources represented more than 80 percent of the sulfur produced in all forms worldwide. This was a less costly approach than collecting sulfur combustion products after the fuel was burned. Second, the United States became more dependent on oil imported from abroad and from Alaska (Prudhoe Bay, Alaska). These new sources of oil had higher concentrations of sulfur than oil recovered from wells in the contiguous 48 States. Sulfur recovered from metallurgical processes, natural gas production (Natural gas), and oil refining has come to dominate U.S. sulfur production.
Sulfur mining by Frasch process
Except for the Frasch process, the technologies for the non-mandated production of sulfur from native sulfur and sulfide mineral deposits are common, conventional mining practices. Frasch mining, which was developed by Herman Frasch in 1894, is an in-situ melting process for extracting sulfur from native sulfur-bearing limestone that caps salt domes found under the waters of the Gulf of Mexico and along the Gulf Coast area.The Frasch process introduces three concentric tubes into the underground sulfur deposit. Superheated water (165 °C, 3 MPa) is injected into the deposit via the outermost tube. Sulfur, which has a melting point of 115 °C, melts and flows into the middle tube. Water pressure alone is unable to force the sulfur to the surface due to the molten sulfur's greater density, so hot air is introduced via the innermost tube to froth the sulfur, making it less dense, and pushing it to the surface.At the surface, the sulfur foam is de-aerated and processed to remove impurities. The molten sulfur is then delivered to vats to solidify. In this way, blocks of pure sulfur, which can weigh more than one ton, are obtained.
Mining of native sulfur and pyrite deposits
The mining of pyrite (iron sulfide) deposits and elemental sulfur deposits (that are not amenable to the Frasch process) use open pit and underground mining methods. High- to medium-grade ore from these deposits can be roasted directly and the resulting sulfur dioxide gas is converted to sulfuric acid. Low-grade ores are treated by a variety of processes, which include agglomeration, direct melting, distillation, flotation, and solvent extraction to produce elemental sulfur. These methods generally are more costly than the Frasch process and they have difficulty competing with the mandated production of sulfur from crude oil and natural gas.
As shown in Figure 1, the recovery of sulfur from pyrites has diminished almost to extinction.
Sulfur recovery from flue gases containing sulfur dioxide
The technology of choice for capturing gaseous sulfur dioxide depends upon the concentration of sulfur dioxide in the overall gas stream. The combustion flue gases gases from power plants that burn sulfur-containing coal is rather low and the technology of choice is known as Flue Gas Desulfurization (FGD) which utilizes various types of scrubbers. The scrubber product is usually a solid compound of calcium (i.e., calcium sulfite and calcium sulfate). With additional processing, some of this material can be made suitable as a substitute for natural gypsum.
The pyrometallurgical processing of metallic sulfide ores generate highly concentrated sulfur dioxide gas. These sulfur resources are ideal for production of sulfuric acid, which is often used in other processes in the same plant that made the acid. For example, copper producers use sulfuric acid as a leachate in the solvent extraction process that recovers copper from very low-grade ores.
More recent production data (2005 – 2009)
The table below presents more recent data on the worldwide proiduction of sulfur from various sources. It should be noted that the vast majority of the 67,500,000 metric tons of sulfur produced worldwide in 2009 was by-product elemental sulfur from petroleum refining and natural gas processing plants.Summary
Experience indicates that human ingenuity as applied to minerals has had remarkable success in extracting elements from the Earth. In the case of sulfur, demand has increased steadily, but the price trend has been relatively flat since 1970. Technological change has resulted in increasing the number of production sources and has also increased the production of sulfur. Consequently, there is no foreseeable shortage of sulfur for the world economy. Sulfur supplies probably will continue to increase as the worldwide effort to control sulfur emissions continues to expand.
Further reading
- D.A. Buckingham, 1991. Availability of elemental sulfur and pyrite concentrate – Market economy countries, U.S. Bureau of Mines Information Circular 9106, 16 p.
- D.A. Buckingham and J.A. Ober, 2002. U.S. Geological Survey, Sulfur statistics, in T.D. Kelly, D.A. Buckingham and C.A. DiFrancesco.
- T.D. Kelly, G.R. Matos, D.A. Buckingham, C.A. DiFrancesco and K.E. Porter, 2001. Historical studies for mineral commodities in the United States U.S. Geological Survey, Data Series 140.
- Ober, J.A., 1998, Sulfur, in Metals and minerals: U.S. Geological Survey Minerals Yearbook 1998, Vol. I, pp. 75.1-75.17.
- Ober, J.A., 2000. Sulfur: U.S. Geological Survey Mineral Commodity Summaries 2000, pp. 164-165.
- U.H.F.Sander, H. Fischer, U. Rothe and R. Kola (1984), Sulphur, sulphur dioxide, and sulphuric acid, British Sulphur Corporation Ltd., 415 pp.
- U.S. Bureau of Mines, 1927-34. Sulfur, in Mineral resources of the United States, 1924-31, U.S. Bureau of Mines, variously paginated.
- U.S. Bureau of Mines, 1933-96. Sulfur, in Metals and minerals, U.S. Bureau of Mines Minerals Yearbook, 1932-94, variously paginated.
- U.S. Bureau of Mines, 1985, Mineral facts and problems, U.S. Bureau of Mines Bulletin 675, pp. 783-798.
- U.S. Geological Survey, 1901-27. Sulfur, in Mineral resources in the United States 1900-23, U.S. Geological Survey, variously paginated.
- U.S. Geological Survey, 1997-2000. Sulfur, in Metals and minerals, U.S. Geological Survey Minerals Yearbook, 1995-98, variously paginated.