Orchidaceae: The Largest Family of Flowering Plants
Orchidaceae, a family of monocotyledonous, angiospermic flowering plants, isthe most species-rich familyin the world withapproximately 19,000-24,000 species. The family is divided into six subfamilies: Orchidoideae, Epidendroideae, Cypripediodeae, Vanilloideae, and Apostasiodeae. Its varied vegetative and floral morphology confers a high taxonomic and ornamental importance, capturing the interest of scientists and horticulturists. In addition, the wide diversity of habitats they occupy and the epiphytic growthof most species make orchids a group of ecological importance. Orchids are widely distributed in the tropics and sub-tropics of Asia (Fig. 1), Africa, Oceania, North, Central(Fig. 2) and South Americas, (Fig. 3) and temperate regions of Asia and Europe.
 Source: William Cetzal-Ix, own work Source: William Cetzal-Ix, own work
|
| Fig. 1: Orchid species in Asia |
 Source: William Cetzal-Ix, own work Source: William Cetzal-Ix, own work
| |
|
 Source: William Cetzal-Ix, own work Source: William Cetzal-Ix, own work
|
| Fig. 3: Orchid species in South America |
 Source: William Cetzal-Ix, own work Source: William Cetzal-Ix, own work
|
| Fig 4: Orchid fruit diversity |
Contents
Distribution
Orchids arefound in almost all terrestrial ecosystems, except at the poles and deserts; however, the greatest diversityoccurs in tropical regions where lush rainforest vegetation, temperature, and high humidity predominate.
Pollination
Many plants have co-evolvedcomplex interelationships with their pollinators. Plants attractpollinators by offering areward such aspollen or nectar. Pollen containsprotein, starch, oils and other nutrients whereasnectaris a sugary liquid. Nectar isproduced by floral structures called nectariesto enticeinsects,birds, or other pollinators to visit a flower so that theycan pick up pollen that can be carried to another conspecific flower. Secondary attractants, such asvisual (color) or olfactory (smells) cues, can direct pollinators topollen and nectar.
| Fig 5: Illegal trade of wild orchids. |
The Orchidaceae is among the largest plant families and orchid species are known for their spectacular floral diversity andadaptations to attract pollinators. A group of orchids are self-pollinated (autogamous),a few are pollinated by birds, but most orchid species are pollinated by insects (Figs 6 and 7). Some studies support the idea that natural selection is the main process behind the evolution of floral orchids and orchid-pollinator relation and this relationship is generally seen as highly asymmetric, because most evolution occurs on the side of the plants. It has been estimated that one third of orchid species are pollinated by food deception and presumably other 400 species by sexual deception (e.g., attracting pollinators by mimicking female mating signals), suggesting that pollination by deception in orchids a highly successful evolutionary strategy (Cozzolino & Widmer 2005). Pollination for food deception (e.g., attracting pollinators by imitating reward signals) has evolved repeatedly in different groups of angiosperms, but is typically restricted to a few species per family. A common trick for food is the presence of false nectaries or points with nectar; this is usually combined with a honey-like smell, which tends to attract bees and flies. Similar is the production of foul smells, along with the colors that simulate rotting meat (van der Pijl & Dodson 1996).
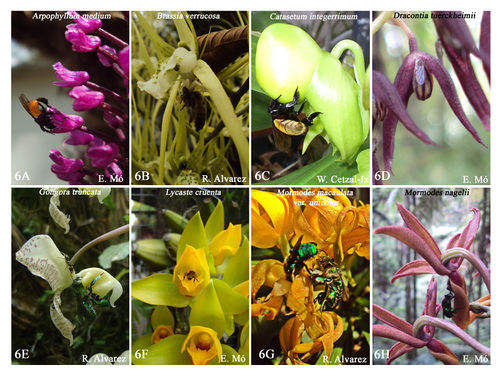 Source: William Cetzal-Ix, own work Source: William Cetzal-Ix, own work
|
| Fig 6: Pollinators and visitors of orchids. |
Several studies (Peakall & Beattie 1996; Johnson & Nilsson 1999; Ayasse et al. 2000) have investigatedthe evolution, causes, and consequences of pollination by deception in orchids andtested different hypotheses to explain its occurrence. 1) The “low-density” hypothesis suggests that pollination by deception could develop whenplant population densities are insufficient to maintain the interest of the pollinator, besides the reward is a superfluous expense and it undertakes best for other plant functions. But a consequence of this hypothesis is that to loss of interest the pollinator, could be promote the autogamy and the shortage of local pollinators. 2) The “pollinia” hypothesisimplies that the pollinia allows a high pollination success with individual visits of insects that do not need a reward to pollinators. 3) The “pollinia-loss” hypothesis implies that deception has evolved in plants due to the specialization of pollinators, where increased fitness has been achieved by reducing pollinia is lost for inconstant pollinators. These hypotheses are not mutually exclusive, and probably different mechanisms act together or individually in the evolution of pollination by deception in orchids.
 Source: William Cetzal-Ix, own work Source: William Cetzal-Ix, own work
|
| Fig 7: Pollinators and visitors of orchids. |
Some authors consider that unrewarded pollination in orchids is an attempt toreducegeitonogamy, the pollination of a flower with pollen from a different flower produced by the same plant. Others explain the presence of pollination by deception as an attempt to the maintenance of a balance between seed production and energy cost of nectar. According to this argument, the resources used to produce rewards could more effectively be used toincreasefruit production and survivalof the plant.
Moreover, studies have demonstrated thatorchid population structureis highly influenced by pollinator behavior. Pollinators foragingin a patch of plants with rewards tend to visit neighboring plants. Pollinators can be deceived into leavingthe patch immediately, especially whenflowers are pollinated by sexual deception. Pollinator behavioraffectsout crossing opportunities and the frequency and distanceover which gene flow occurs which hasinfluenced thethe genetic population structure of many orchids. Genetic differentiation among populations is higher for orchids that producerewards compared with orchids that rely ondeception, suggesting that gene flowbetween populations is high in deceptive orchids.
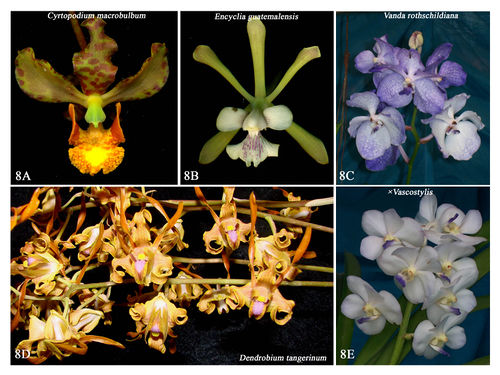 Source: William Cetzal-Ix, own work Source: William Cetzal-Ix, own work
|
| Fig 8: Orchids from Mexico and Asia |
Phylogenetic studies in Vanilloideae and Cypripedioideae indicate that the lack of nectar could represent the primitive state in the Orchidaceae (Cozzolino & Widmer 2005). But the question of whetherancestral orchids offered pollen as a reward or were pollinated by deceit remains unanswered. It is well known that pollination by deception has evolved several times independently in many species, in the main clade of orchids. Dafni (1987) proposed a scenario for the Mediterranean Orchidinae in which deceit pollination for food or sexual, evolved independently from a nectariferous ancestor. However, phylogenetic analysis and mapping strategies for pollination in a phylogeny of Orchidinae did not support this hypothesis, suggesting that pollination by deceit food is ancestral and predominant strategy in this tribe, and pollination reward evolved more than once regardless of pollination by deception food, as previously suggested.
Pollination by sexual deceptionalso evolved from food deceptive in the genus Ophrys and independently in Orchis galilaea Schltr. This is evidenced similarly in the southern African genus Disa offering nectar reward their pollinators and species pollinated by sexual deception evolved from an ancestor of deception for food. In South America, pollination by sexual deception evolved independently in Trigonidium and Mormolyca and probably in Oncidiinae sub-tribe. The most spectacular radiation of orchids pollinated by sexual deception occurred in Australia, where this pollination strategy evolved several times in nine genera. Currently unknown whether on pollination by reward or deception food have evolved from sexual deception.
Evolutionary Radiation
The adaptive capacityofmembers of the Orchidaceae has played a significant role in the evolutionary radiation of this group,leading to the rapid diversificationof numerous new species displaying extensive vegetative and floral morphological complexity.Thus, theOrchidaceaeis an interesting group with respect to investigations in studies on systematics, phylogenetics, biogeography, evolution, ecology, conservation, and pollination biology.
Conservation
Orchids are one of the most vulnerable floral groups in tropical and subtropical countries with a large number of species experiencingserious conservation risk. Global warming,climate change,and several related anthropogenic factors such as unrestricted harvesting for illegal trades in orchid nurseries, gardening industries, and private collectors are responsible for rapid decline of orchid diversity reported from the continents of Asia, Africa and Latin America, the regions with the greatest Orchid species diversity.
Possible solutions to counter the decline of species is to increase the number of protected natural areas in regions with high orchid diversity, encourage both formal and informal environmental education, attract ex situ collections, establish germplasm banks, promote laws to be implemented to curb the illegal trade, and motivate communities to see their forests as a source of environmental services. Furthermore, promoting studies on the natural history, reproductive biology, demography, genetic diversity, relationships with mycorrhizae, and ethnobotanical uses of orchids as well as reintroducing individual species propagated by artificial means to natural environments could also help conserve species.
Due to the primary importance of these predominantly epiphytic species in several tropical and sub-tropical ecosystems we have suggested that the Orchid members be treated as flagship species in such vulnerable and unique ecosystems for overall assessment of the health of these specific habitats.
References and Further Reading
- American Orchid Society. https://www.aos.org/Default.aspx?id=50
- Ayasse, M., Schiestl, F., Paulus, H., Lofstedt, Hansson, B., Ibarra, F., & Francke, W. 2000. Evolution of reproductive strategies in the sexual deceptive Orchid Ophrys sphegodes: How does flower-specific variation of odor signals influence reproductive success? Evolution 54 (6): 1995-2006
- Carnevali, G., W. Cetzal-Ix, R. Balam, C. Leopardi & G.A. Romero-González. 2013. A combined evidence phylogenetic re-circumscription and a taxonomic revision of Lophiarella (Orchidaceae: Oncidiinae). Systematic Botany 38(1): 46–63.
- Cetzal-Ix, W. & G. Carnevali. 2010. A revision of Cohniella Pfitzer (Orchidaceae) in Mexico. The Journal of the Torrey Botanical Society 137(2-3): 180–213.
- Cetzal-Ix, W., Alvarez-Mora, R., Basu, S. K., Cosme-Pérez, J. and Noguera-Savelli, E. [2014]. Orchid fruit diversity at Puebla Mexico: A new insight into the biodiversity of a fragmented ecosystem with need for conservation and potential for horticultural exploitations in future. In: Nandwani, D. (edited). Role of horticulture in biodiversity conservation. Springer- Science + Business Media, Dordrecht, The Netherlands. Chapter 9. pp. XX-XX. 450 p. Aug. 31. http://www.springer.com/life+sciences/agriculture/book/978-3-319-06903-6
- Cozzolino, S. and Widmer, A. 2005. Orchid diversity: an evolutionary consequence of deception? Trends in Ecology and Evolution 20(9):487-494.
- Dafni, A. 1987. Pollination in Orchis and related genera: evolution from reward to deception. In Orchid Biology, Reviews and Perspectives (Arditti, J., ed.), pp. 79-104, Cornell University Press.
- Darwin, C. 1862. On the various contrivarences by which Brithish and foreign orchids are fertilized by insects. John Murray, London.
- Fontúrbel, F. & D. Mondaca. (2000). Coevolución insecto–planta en la polinización. Revista Estudiantil de Biología, 1 (1): 18–27.
- Gardiner, L.M., Bone, R. & N.M. Kilgallen. 2012. Orchids and eMonocot – assembling research resources and facilitating collaborative taxonomy online. Lankesteriana 13(1-2): 33-37.
- Johnson, D. & L. Nilsson. 1999. Pollen carryover, geitonogamy, and the evolution of deceptive pollination systems in orchids. Ecology 80: 2607-2619.
- Nilson, A.1992. Orchid Pollination Biology. TREE 7(8):255-258.
- Pant, B. 2013. Medicinal orchids and their uses: Tissue culture a potential alternative for conservation. African Journal of Plant Science 7(10): 448-467.
- Pupulin, F. 2012. Toward a global orchid taxonomic network. Lankesteriana 13(1-2): 75-91.
- Peakall, R. & A. Beattie. 1996. Ecological and genetic consequences of pollination by sexual deception in the orchid Caladenia tentaculata. Evolution 50: 2207-2220.
- Scagel R., Bandoni R., Rouse G., Schofield W., Stein J. & T. Taylor. (1977). El Reino vegetal: los grupos de plantas y sus relaciones evolutivas. Ediciones Omega, Barcelona, pp 570, 573-575.
- Swarts, N.D. & K.W. Dixon. 2009. Terrestrial orchid conservation in the age of extinction. Annals of Botany 104: 543-556.
- Swarts, N.D. & K.W. Dixon. 2009. Perspectives on orchid conservation in botanic gardens. Trends in Plant Science 14(11): 590-598.
- Seaton, P.T., J.P. Kendon, H.W. Pritchard, D.M. Puspitaningtyas & T.R. Marks. 2012. Orchid conservation: the next ten years. Lankesteriana 13(1-2): 93-101.e
- Epidendra. The Global Orchid Taxanoic Network.http://www.epidendra.org/
- eMonocot. An online resource for monocot plants.http://emonocot.org/
- The Orchid Specialist Group. IUCN. https://www.iucn.org/about/work/programmes/species/who_we_are/ssc_specialist_groups_and_red_list_authorities_directory/plants/orchid_specialist_group/
- van der Pijl, L. & C. Dodson. 1996. Orchids Flowers, Their Pollination and Evolution, University of Miami Press.

