Nuclear fuel cycle
This EOE article is adapted from an information paper published by the World Nuclear Association (WNA). WNA information papers are frequently updated, so for greater detail or more up to date numbers, please see the latest version on WNA website (link at end of article).
Contents
- 1 Introduction The closed nuclear fuel cycle. The open nuclear fuel cycle. the fast breeder nuclear fuel cycle The various activities associated with the production of electricity from nuclear reactions are referred to collectively as the nuclear fuel cycle. The nuclear fuel cycle starts with the mining of uranium (Nuclear fuel cycle) and ends with the disposal of nuclear waste. With the reprocessing of used fuel as an option for nuclear energy, the stages form a true cycle.
- 2 Uranium
- 3 Uranium Mining
- 4 Uranium Milling
- 5 Conversion
- 6 Enrichment
- 7 Fuel fabrication
- 8 Power generation
- 9 Used fuel
- 10 Used fuel storage
- 11 Reprocessing
- 12 Uranium and Plutonium Recycling
- 13 Used fuel disposal
- 14 Wastes
- 15 Further Reading
Introduction The closed nuclear fuel cycle. The open nuclear fuel cycle. the fast breeder nuclear fuel cycle The various activities associated with the production of electricity from nuclear reactions are referred to collectively as the nuclear fuel cycle. The nuclear fuel cycle starts with the mining of uranium (Nuclear fuel cycle) and ends with the disposal of nuclear waste. With the reprocessing of used fuel as an option for nuclear energy, the stages form a true cycle.
Uranium
Uranium is a slightly radioactive metal that occurs throughout the Earth's crust. It is about 500 times more abundant than gold and about as common as tin. It is present in most rocks and soils as well as in many rivers and sea water. It is, for example, found in concentrations of about four parts per million (ppm) in granite, which makes up 60% of the Earth's crust. In fertilizers, uranium concentration can be as high as 400 ppm (0.04%), and some coal deposits contain uranium at concentrations greater than 100 ppm (0.01%). Most of the radioactivity associated with uranium in nature is in fact due to other minerals derived from it by radioactive decay processes, and which are left behind in mining and milling.
There are a number of areas around the world where the concentration of uranium in the ground is sufficiently high that extraction of it for use as nuclear fuel is economically feasible. Such concentrations are called ore.
Uranium Mining
Both excavation and in situ techniques are used to recover uranium ore. Excavation may be underground or open pit mining.
In general, open pit mining is used where deposits are close to the surface and underground mining is used for deep deposits, typically greater than 120 meters deep. Open pit mines require large holes on the surface, larger than the size of the ore deposit, since the walls of the pit must be sloped to prevent collapse. As a result, the quantity of material that must be removed in order to access the ore may be large. Underground mines have relatively small surface disturbance and the quantity of material that must be removed to access the ore is considerably less than in the case of an open pit mine.
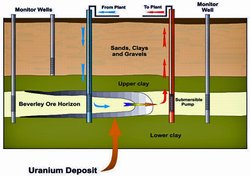
An increasing proportion of the world's uranium now comes from in situ leaching (ISL), where oxygenated groundwater is circulated through a very porous orebody to dissolve the uranium and bring it to the surface. ISL may be with slightly acid or with alkaline solutions to keep the uranium in solution. The uranium is then recovered from the solution as in a conventional mill (see below).
The decision as to which mining method to use for a particular deposit is governed by the nature of the orebody, and safety and economic considerations.
In the case of underground uranium mines, special precautions, consisting primarily of increased ventilation, are required to protect against airborne radiation exposure.
Uranium Milling
 In situ uranium leaching. (Source: World Nuclear Association)
In situ uranium leaching. (Source: World Nuclear Association) A uranium mill is a chemical plant that extracts uranium from mined ore. Most mining facilities include a mill, although where mines are close together, one mill may process the ore from several mines. At conventional mills, the ore arrives via truck and is crushed, ground and leached. In most cases, sulfuric acid is the leaching agent, but alkaline leaching can also be done. The leaching agent extracts some 90 to 95 percent of the uranium from the ore. The uranium is then removed from this solution, precipitated and dried. Mills are typically in areas of low population density, and they may process ores from mines within 50 kilometers (30 miles). Milling produces a uranium oxide concentrate that is then shipped from the mill. It is sometimes referred to as yellowcake and generally contains more than 80% uranium. The original ore may contain as little as 0.1% uranium, or less.
As mentioned above, in situ leach (ISL) facilities are one means of extracting uranium from underground. The leach solution is pumped from the orebody underground to the processing plant, and ion exchange separates the uranium from the solution. it is then purified and dried.
in either case it is usually shipped from the mine in 200-litre drums packed into a standard shipping container.
Potential Industrial Hazards and Wastes from Milling
Because the uranium is not enriched, there is no criticality hazard and little fire or explosive hazard for it. If solvent extraction is used in the mill, the hydrocarbons involved process do present a fire hazard, however. The primary hazards associated with milling operations are occupational hazards found in any metal milling operation that uses chemical extraction plus the chemical toxicity of the uranium itself.
Radiological hazards are low at these facilities as uranium has little penetrating radiation and only moderate non-penetrating radiation. The primary radiological hazard is due to the presence of radium in the uranium decay chains and the production of radon gas from the decay of radium and radon progeny (short-lived radon decay products). This is managed by ventilation.
The remainder of the ore, containing most of the radioactivity and nearly all the crushed and ground rock material, becomes tailings, which are emplaced in engineered facilities near the mine (often in a mined-out pit). Tailings contain long-lived radioactive materials in low concentrations and toxic materials such as heavy metals; however, the total quantity of radioactive elements is less than in the original ore, and their collective radioactivity will be much shorter-lived. These materials need to be isolated from the environment.
Uranium mill tailings, contain most of the progeny (decay products) of uranium, and from high-grade ore may be a significant source of radon and radon progeny releases to the environment. The hazards from radon involve inhalation of radon progeny that may be deposited in the respiratory tract. Alpha radiation could be emitted into those tissues and can pose a cancer risk to workers in the absence of proper ventilation.
Conversion
The product of a uranium mill is not directly usable as a fuel for a nuclear power reactor. Additional processing, generally referred to as enrichment, is required for most kinds of reactors. This process requires uranium to be in gaseous form and the way this is achieved is to convert it to uranium hexafluoride, which is a gas at relatively low temperatures.
Hence the next step in the fuel cycle is conversion into pure uranium hexafluoride (UF6) suitable for use in enrichment operations. During this conversion, impurities are removed and the uranium is combined with fluorine to create the UF6 gas. The UF6 is then pressurized and cooled to a liquid. In its liquid state it is drained into 14-tonne cylinders where it solidifies after cooling for approximately five days. The UF6 cyclinder, in the solid form, is then shipped to an enrichment plant. UF6 is the only uranium compound that exists as a gas at a suitable temperature.
As with mining and milling, the primary risks associated with conversion are chemical and radiological. Strong acids and alkalis are used in the conversion process, which involves converting the yellowcake (uranium oxide) powder to very soluble forms, leading to possible inhalation of uranium. The main hazard of this stage of the fuel cycle is the use of highly corrosive hydrogen fluoride.
Enrichment
Natural uranium consists, primarily, of a mixture of two isotopes (atomic forms) of uranium. Only 0.7% of natural uranium is "fissile", or capable of undergoing fission in a normal reactor. Fission is the process by which energy is produced in a nuclear power reactor. The fissile isotope of uranium is uranium-235 (235U); the remainder is mostly uranium-238 (238U).
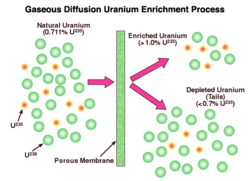
In the most common types of nuclear reactors, a higher-than-natural concentration of 235U is required. The enrichment process produces this higher concentration, typically between 3.5% and 5% 235U, by removing over 85% of the 238U. This is done by separating gaseous uranium hexafluoride into two streams, one being enriched to the required level and known as low-enriched uranium. The other stream is progressively depleted in 235U and is called 'tails'.
There are two enrichment processes in large-scale commercial use, each of which uses uranium hexafluoride as feed: gaseous diffusion and gas centrifuge. They both use the physical properties of molecules, specifically the 1% mass difference, to separate the isotopes. The product of this stage of the nuclear fuel cycle is enriched uranium hexafluoride, which is reconverted to produce enriched uranium oxide.
Gaseous diffusion
In the gaseous diffusion enrichment plant, the solid uranium hexafluoride (UF6) from the conversion process is heated in its container until it becomes a liquid. The container becomes pressurized as the solid melts and UF6 gas fills the top of the container. The UF6 gas is slowly fed into the plant’s pipelines where it is pumped through special filters called barriers or porous membranes. The holes in the barriers are so small that there is barely enough room for the UF6 gas molecules to pass through. The isotope enrichment occurs when the lighter UF6 gas molecules (with the U-234 and U-235 atoms) tend to diffuse faster through the barriers than the heavier UF6 gas molecules containing U-238. One barrier isn’t enough, though. It takes many hundreds of barriers, one after the other, before the UF6 gas contains enough uranium-235 to be used in reactors. At the end of the process, the enriched UF6 gas is withdrawn from the pipelines and condensed back into a liquid that is poured into containers. The UF6 is then allowed to cool and solidify before it is transported to fuel fabrication facilities where it is turned into fuel assemblies for nuclear power reactors.
The primary hazard in gaseous diffusion plants include the chemical and radiological hazard of a UF6 release, and the potential for mishandling the enriched uranium, which could create a criticality accident (inadvertent nuclear chain reaction).The diffusion enrichment process is very energy-intensive and is obsolete, so will be largely abandoned by 2015.
Gas centrifuge
The gas centrifuge uranium enrichment process uses a large number of rotating cylinders in series and parallel formations. Centrifuge machines are interconnected to form trains and cascades. In this process, UF6 gas is fed into a series of vacuum tubes, each containing a rotor about one to two metres long and 15-20 cm diameter. When the rotors are spun rapidly, at 50,000 to 70,000 rpm, the heavier molecules with U-238 increase in concentration towards the cylinder’s outer edge. There is a corresponding increase in concentration of U-235 molecules near the centre. These concentration changes are enhanced by inducing the gas to circulate axially within the cylinder.
The stream that is slightly enriched in U-235 is withdrawn and fed into the next higher stage, while the slightly depleted stream is recycled back into the next lower stage. Eventually enriched and depleted uranium are drawn from the cascade at the desired assays. To obtain efficient separation of the two isotopes, centrifuges rotate at very high speeds, with the outer wall of the spinning cylinder moving at between 400 and 500 metres per second to give a million times the acceleration of gravity.
Although the capacity of a single centrifuge is much smaller than that of a single diffusion stage, its capability to separate isotopes is much greater. Centrifuge stages normally consist of a large number of centrifuges in parallel. Such stages are then arranged in series in cascades similar to those for diffusion. In the centrifuge process, however, the number of stages may only be 10 to 20, instead of a thousand or more for diffusion. Most world enrichment capacity will be centrifuge within a few years.
Fuel fabrication
Nuclear fuel fabrication makes the enriched UF6 into fuel for nuclear power reactors. Reactor fuel is generally in the form of ceramic pellets. These are formed from pressed uranium oxide that is sintered (baked) at a high temperature (over 1400ƒ C). The pellets are then encased in metal tubes to form fuel rods, which are then arranged into a fuel assembly and ready for introduction into a reactor. The dimensions of the fuel pellets and other components of the fuel assembly are precisely controlled to ensure consistency in the characteristics of fuel bundles.
In a fuel fabrication plant, great care is taken with the size and shape of processing vessels to avoid criticality (a limited chain reaction releasing radiation). With low-enriched fuel, criticality is highly unlikely, but in plants handling special fuels for research reactors, this is a vital consideration.
Power generation
Inside a nuclear reactor the nuclei of uranium-235 atoms split (fission) and, in the process, release energy. This energy is used to heat water and turn it into steam. The steam is used to drive a turbine connected to a generator that produces electricity. Some of the uranium-238 in the fuel is turned into plutonium in the reactor core. The main plutonium isotope is also fissile and it yields about one third of the energy in a typical nuclear reactor. The fissioning of uranium is used as a source of heat in a nuclear power plant in the same way that the burning of coal, gas or oil is used as a source of heat in a fossil fuel power plant.
An issue in operating reactors and hence specifying the fuel for them is fuel burn-up. This is measured in gigawatt-days per tonne and its potential is proportional to the level of enrichment. Hitherto a limiting factor has been the physical robustness of fuel assemblies, and hence burn-up levels of about 40 GWd/t have required only around 4% enrichment. But with better equipment and fuel assemblies, 55 GWd/t is possible (with 5% enrichment), and 70 GWd/t is in sight, though this would require 6% enrichment. The benefit of this is that operation cycles can be longer – around 24 months – and the number of fuel assemblies discharged as used fuel can be reduced by one third. Associated fuel cycle cost is expected to be reduced by about 20%.
As with as a coal-fired power station about two thirds of the heat is dumped, either to a large volume of water (from the sea or large river, heating it a few degrees) or to a relatively smaller volume of water in cooling towers, using evaporative cooling (latent heat of vapourisation).
Used fuel
With time, the concentration of fission fragments and heavy elements formed in the same way as plutonium in a fuel bundle will increase to the point where it is no longer practical to continue to use the fuel. So, after 12-24 months the 'spent fuel' is removed from the reactor. The amount of energy produced from a fuel bundle varies with the type of reactor and the policy of the reactor operator.
Typically, some 46 million kilowatt-hours of electricity are produced from one tonne of natural uranium. The production of this amount of electrical power from fossil fuels would require the burning of over 20,000 tonnes of black coal or 8.5 million cubic metres of gas.
Used fuel storage
When removed from a reactor, a fuel bundle will be emitting both radiation, principally from the fission fragments, and heat. Used fuel is unloaded into a storage pond immediately adjacent to the reactor to allow the radiation levels to decrease. In the ponds, the water shields the radiation and absorbs the heat. Used fuel is held in such pools for several months to several years.
Depending on policies in particular countries, some used fuel may be transferred to central storage facilities. Ultimately, used fuel must either be reprocessed or prepared for permanent disposal.
Reprocessing
Used fuel is about 95% uranium-238 but it also contains up to 1% uranium-235 that has not fissioned, about 1% plutonium and 3% fission products, which are highly radioactive, with other transuranic elements formed in the reactor. In a reprocessing facility the used fuel is separated into its three components: uranium, plutonium and waste, containing fission products. Reprocessing enables recycling of the uranium and plutonium into fresh fuel, and produces a significantly reduced amount of waste (compared with treating all used fuel as waste).
Uranium and Plutonium Recycling
The uranium from reprocessing, which typically contains a slightly higher concentration of 235U than occurs in nature, can be reused as fuel after conversion and enrichment, if necessary. The plutonium can be directly made into mixed oxide (MOX) fuel, in which uranium and plutonium oxides are combined.
In reactors that use MOX fuel, plutonium substitutes for the 235U found in normal uranium oxide fuel.
Used fuel disposal
At present, there are no disposal facilities (as opposed to storage facilities) in operation in which used fuel, not destined for reprocessing, and the waste from reprocessing can be placed. Although many technical issues related to disposal have been addressed, there is currently no pressing technical need to establish such facilities, as the total volume of such wastes is relatively small. Further, the longer the wastes are stored, the easier they are to handle due to the progressive diminution of radioactivity. There is also a reluctance to dispose of used fuel because it represents a significant energy resource that could be reprocessed at a later date to allow recycling of the uranium and plutonium. Technical issues aside, opponents of nuclear power point to the lack of a long term disposal option as a significant political and public credibility problem for the industry.
A number of countries are carrying out studies to determine the optimum approach to the disposal of spent fuel and wastes from reprocessing. The general consensus favors its placement into deep geological repositories, initially recoverable.
Wastes
Nuclear waste management is a critical part of the fuel cycle. Wastes from the nuclear fuel cycle are categorized as high-, medium- or low-level wastes by the amount of radiation that they emit. These wastes come from a number of sources and include:
- low-level waste produced at all stages of the fuel cycle
- intermediate-level waste produced during reactor operation and by reprocessing
- high-level waste, which is waste containing fission products from reprocessing, and in many countries, the used fuel itself.
The enrichment process leads to the production of much 'depleted' uranium, in which the concentration of uranium-235 is significantly less than the 0.7% found in natural uranium. Small quantities of this material, which is primarily uranium-238, are used in applications where high-density material is required, including radiation shielding and in the production of MOX fuel. While 238U is not fissile, it is a low specific activity radioactive material and some precautions must, therefore, be taken in its storage or disposal.
Material balance in the nuclear fuel cycle: The following figures make various assumptions (see footnote) but may be regarded as typical for the operation of a 1000 MWe nuclear power reactor:
| Mining | 20 000 tones of 1% uranium ore |
| Milling | 230 tonnes of uranium oxide concentrate (with 195 t U) |
| Conversion | 288 tonnes UF6 (with 195 t U) |
| Enrichment | 35 tonnes UF6 (with 24 t enriched U) - balance is 'tails' |
| Fuel fabrication | 27 tonnes UO2 (with 24 t enriched U) |
| Reactor operation | 8640 million kWh (8.64 TWh) of electricity at full output |
| Used fuel | 27 tonnes containing 240kg plutonium, 23 t uranium (0.8% U-235), 720kg fission products, also transuranics. |
| Concentrate is 85% U, enrichment to 4% 235U with 0.25% tails assay - hence 140,000 SWU required, core load 72 tU, refuelling so that 24 tU/yr is replaced. Operation: 45,000 MWday/t (45 GWd/t) burn-up, 33% thermal efficiency. (In fact a 1000 MWe reactor cannot be expected to run at 100% load factor - 90% is more typical best, so say 7.75 TWh/yr, but this simply means scaling back the inputs accordingly.) | |
Uranium concentrations are sometimes expressed in terms of U3O8 content (U3O8 is a mixture of two uranium oxides approximately as they occur in nature). Pure U3O8 product contains about 85% uranium metal.
Further Reading
- WNA paper on Nuclear fuel cycle
- World Nuclear Association
- The Nuclear fuel cycle, The U.S. Nuclear Regulatory Commission
| Disclaimer: This article is taken wholly from, or contains information that was originally published by, the U.S. Nuclear Regulatory Commission. Topic editors and authors for the Encyclopedia of Earth may have edited its content or added new information. The use of information from the U.S. Nuclear Regulatory Commission should not be construed as support for or endorsement by that organization for any new information added by EoE personnel, or for any editing of the original content. |