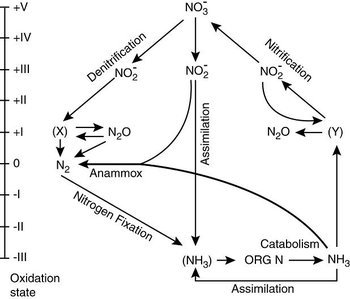
Marine nitrogen cycle
Nitrogen (N) is an essential macronutrient the non-availability of which in suitable form or concentration often limits biological production both in the terrestrial and marine environments. It is a polyvalent element that occurs in oxidation states ranging from –3 to +5. Molecular nitrogen (N2), the dominant constituent of our atmosphere, is the most abundant form of nitrogen on Earth. However, the triple N-N bond makes N2 almost inert, and only a few microorganisms have the capability to utilize (fix) N2, converting it to the more easily utilizable combined nitrogen forms – initially ammonia (NH3), or its protonated species, ammonium (NH4+) that is terminally oxidized to nitrate (NO3-) by nitrifying bacteria. Nitrification, a chemoautotrophic process, is carried out by a consortium of bacteria and involves production of intermediates such as nitrite (NO2-), and nitrous oxide (N2O) as a byproduct. Due to the oxidizing nature of the Earth’s surface environment, including most of the oceanic water column, NO3- is by far the most abundant combined nitrogen species in aquatic systems. However, nitrogen occurs in the most reduced form in biological materials and so when primary producers utilize NO3- as the nitrogenous nutrient, it must be reduced to the –3 state; most plants are enzymatically equipped for this purpose. Nitrifiers also oxidize reduced nitrogen released from the decaying organic matter and excreted by organisms. Biological and chemical fixation of N2 that occurs on a smaller scale through lightning must somehow be compensated by N2 production in order to maintain the atmospheric N2 content constant over geological time scales. This is achieved through the process of denitrification that occurs in anaerobic environments. It involves the reduction of NO3- to N2 with NO2-, nitric oxide (NO) and N2O as intermediates. Another pathway of N2 production is the anaerobic ammonium oxidation (anammox; NH4++ NO2- → N2 + 2H2O) which, in contrast to denitrification, is carried by chemoautotrophic bacteria.
Uptake by phytoplankton often results in the nearly complete depletion of inorganic combined forms in sunlit, stratified surface waters of the ocean, whereas sinking of organic debris and its degradation and consequent release of inorganic nitrogen causes the latter to accumulate in the subsurface layers along with other macronutrients such as phosphate. Reflux of NO3- to the euphotic zone through upwelling and vertical mixing largely regulates primary production except in remote areas (high-nutrient low-chlorophyll regimes) such as the equatorial Pacific and the Southern Ocean where low concentration of a micronutrient (iron) appears to limit photosynthesis. In areas characterized by low dissolved inorganic nitrogen (DIN) concentration in surface waters, dissolved organic nitrogen (DON) may be the most abundant form of combined nitrogen and hence an important nitrogen source for both primary and secondary producers. However, most of the DON pool in the ocean is relatively refractory. As in the case of phosphate, NO3- concentration exhibits an inverse relationship with oxygen concentration, and increases downstream of the deep-water flow, being the lowest in the North Atlantic and the highest in the North Pacific.
Rate of N-fixation in the ocean is poorly constrained; its estimate having risen steadily in recent times currently stands at about 135 Tg N a-1 (1 Tg = 1012 g). This is comparable with total inputs of combined nitrogen to the ocean by river runoff (~35 and 45 Tg N a-1 in the dissolved inorganic and particulate organic forms, respectively) and atmospheric deposition (~50 Tg N a-1), a substantial fraction of which is believed to be of anthropogenic (human-made) origin. Of the various loss terms of combined nitrogen from the ocean, emission of N2O to the atmosphere (4-7 Tg N a-1) is quantitatively not important for the oceanic combined nitrogen budget, even though for the atmosphere itself it represents a major source of N2O. The burial rate in the sediments is also quite modest (~25 Tg N a-1). The most important loss terms are sedimentary and water-column denitrification. The former occurs in all parts of the ocean, especially along the continental margins, because anaerobic conditions required for the onset of denitrification are usually reached within a few millimeters to a few centimeters below the sediment-water interface. However, sedimentary denitrification rate is highly variable, and this together with limited measurements makes estimates of sedimentary denitrification highly uncertain (ranging from 180 to 300 Tg N a-1). One would expect the extent of water-column denitrification to be better constrained due to the geographical restriction of vigorous denitrification to just a few well-demarcated sites - within the oxygen minimum zones (OMZs) of the eastern tropical Pacific and the northwestern Indian Ocean (Arabian Sea). However, current estimates of water-column denitrification also vary widely (from 65 to 150 Tg N a-1).
A part of the uncertainty in water-column N2 production arises from the fact that most estimates are based on the deficiency in NO3- within the OMZs relative to the concentration expected from the nearly-constant relative changes in C:N:P:O in seawater due to biological activity (the Redfield Ratios: 106:16:1:138), whereas the recently discovered anammox process supported by measurements of N2/Ar suggests that the extent of N2 production might substantially exceed ‘nitrate deficits’. Although with the lower ends of ranges for both sedimentary and water column denitrification, it is possible that the oceanic combined nitrogen budget could still be in a steady state, it is more likely that in the present day ocean, losses of combined nitrogen significantly exceed inputs. The proponents of the non-steady state believe that the ocean oscillates between a net source and net sink of combined nitrogen on time scales of hundreds to tens of thousand of years, and the associated changes in the combined nitrogen inventory affect the oceanic capacity to sequester atmospheric carbon dioxide (CO2), thereby modulating, in part, climatic (glacial-interglacial) cycles. Those who believe in a balanced budget, argue that oceanic nitrate/phosphate atomic ratio (~15) is quite similar to the “Redfieldian” ratio of 16 in plankton and the intercept of the linear nitrate/phosphate relationship is very close to zero. This in conjunction with the domination of the budget by biological sources and sinks – nitrogen fixation and denitrification, which may be tightly coupled – is expected to favor “homeostasis” that would opposes large imbalances. Thus, it is possible that the present imbalance in combined nitrogen budget could either be a transient phenomenon, or it may represent an increase in the oceanic denitrification rates during the “Anthropocene” due to human activity.
Further Reading
- Codispoti, L. A., Brandes, J. A., Christensen, J. P., Devol, A. H., Naqvi, S. W. A., Paerl, H. W., and Yoshinari, T.: The oceanic fixed nitrogen and nitrous oxide budgets: Moving targets as we enter the anthropocene?, Sci. Mar., 65, 85-105, 2001.
- Gruber, N.: The dynamics of the marine nitrogen cycle and its influence on atmospheric CO2, in: The Ocean Carbon Cycle and Climate, edited by: Follows, M., and Oguz, T., Kluwer Academic, Dordrecht, 97-148, 2004. ISBN: 1402020864