Marine microbial loop
The material presented here tends to resume the literature dealing mainly with the structural description of the microbial loop and discusses some functional aspect in action within the microbial food webs. For more detailed information, the interested readers can refer to the literature listed below.
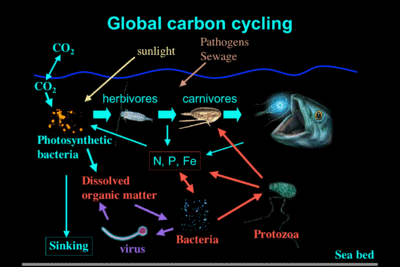
Since Pomeroy (1974), it has been shown that the microbial consortia play a key role in both structure and function of open ocean ecosystems (Azam et al., 1983). Figure 1 shows a cartoon representation of the oceanic global carbon cycling.
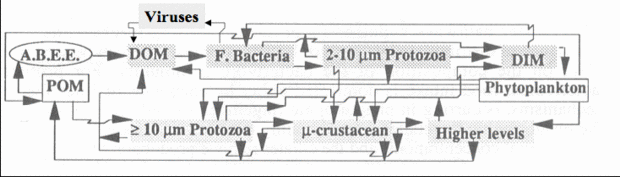
Two major herbivorous and microbial pathways determine transformation and transfer of matters in the ocean (Fig. 2). The relative flux intensity within each pathway depends upon a “competition” between bacteria and the particle grazers’ pathways. Due to the dominance of the bacterial production in oligotrophic environments and in most of the mesoplelagic water column, fluxes are highly diverted towards the pathway n°1. However, it is important to appreciate conditions that determine flux partitioning between these paths.
Transfer pathways hypothesis: In marine ecosystems, the “Microbial Loop” can be distinguished from the “Microbial Food Webs” in that the former likely consists of the pathways relating heterotrophic bacteria to bacterivorus protests (zooflagellates) and Dissolved Organic Matter (DOM), and the latter includes all microbial communities below ca 100 µm including all the ? 10 µm primary producing organisms. Therefore, as much as it is true that the Microbial Loop can mainly act as a carbon sink, the Microbial Food Web is the crucial link for the whole ecosystems. The sink aspect is mostly due to the fact that a considerable amount of Particulate Organic Matter (POC) passes through bacterial production that end up in DOM pools. Four issues are considered:
- Transfer pathways hypothesis – size of primary producers: It can be appreciated that the size of the primary producers is what determines whether a microbial community is going to act mainly as a trophic sink or link in the marine ecosystems. For instance, when cyanobacteria dominate an ecosystem, the primary production is mostly trapped within the Microbial Loop. Under such conditions not more than 6% of the primary production can reach higher trophic levels. Whereas, when > 2 µm phytoplankton dominate, then up to 20% is transferred, which would constitute the total basic supply for the whole system.
- Transfer pathways hypothesis – patchiness: In addition to the size of the primary producers, patchiness in the oligotrophic pelagial is probably the most important feature in the structure and function of the water column ecosystem. We believe that the primary productivity is regulated by small-scale microbial interactions, mainly through feed-backs (“mutualism”) between free-living bacteria and phytoplankton, optimizing the use of mineral nutriments at low concentrations; condition characterizing the oligotrophic environments that represent the most open ocean ecosystems. Patchiness must also be important for protozoan survival, because oligotrophic concentrations of prey (? 20 µg C l-1) hardly support optimal growth conditions (Km) for most microphagous organisms that develop, however, normally in such poor conditions. Therefore, appropriate space distribution of both nutrient and prey appears as essential survival conditions: “hot spots”.
- Transfer pathways hypothesis – protozoan feeding mechanisms: The importance of distributional patterns of bacteria, for instance, can be seen in its effect on the composition of the bacterivorous community. For protistan bacterivores, the food intake is dominated either by encounter or filter feeding mechanisms, often occurring in phagotrophic flagellates and ciliates, respectively. The phagotrophic flagellates (zooflagellates) control mainly the bacterial production, at rather high bacterial concentrations, and the ciliates remove production of > 1 µm both hetero- and autotrophic cells, and to some extent of bacteria. Indeed, open ocean’s ciliates are mostly Polyhymenophorean, multiple mouth surrounding membranelles that have relatively high specific water filtration flux, due to large inter-membranelle spacing, allowing them to survive on nano-sized prey at regular low oligotrophic concentrations. However, one can notice that some polymenophorean ciliates are so small (? 12 µm) that they can retain bacteria as well, while at low concentrations (high water flux through mouth filtration structure) in open ocean, where zooflagellates can become almost inefficient grazers on bacteria at oceanic bulk phase concentrations. The size differential between predator and prey in a microbial food web (in this instance, bacteria and bacterivores) can depend on prey concentrations.
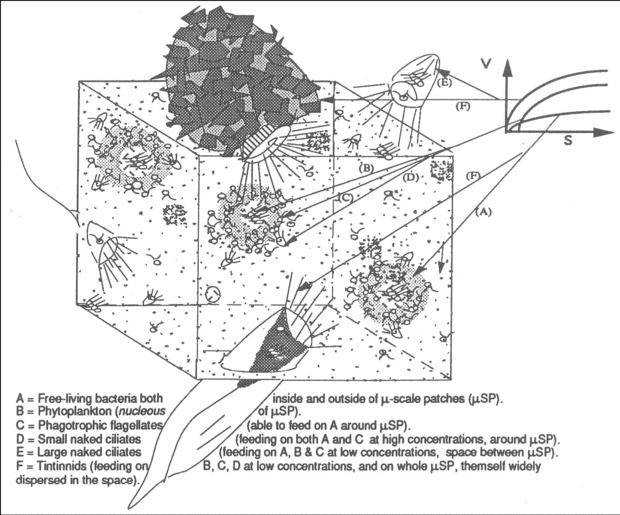
- Transfer pathways hypothesis – spatial organization of food web: with regard to the NH4+ Mjor phytoplnktonic regenerted inorgnic supply, supplied minly by protozon excretion through the gzing of the “extr-biomss” production in both bcteri nd microlge, the bacteria-phytoplankton mutualism depends upen low Km-low Vmax (low flow) and High Km-High Vmax (high flow) uptake systems for NH4+ in free-living bacteria and phytoplankton, respectively. Bacteria could thus be more active than phytoplankton at low NH4+ concentrations (outside of phytoplankton-bacteria “hot spots” or “nutrient spheres”, and vice versa, at high NH4+ concentration (inside of “nutrient spheres” at vicinity of phytoplankton cells). A similar situation seems to exist for the bacterial affinity for their own their own (5’-nucleotidase mediated) orthophosphate liberated within these “hot-spots”. Figure 3 shows a cartoon diagram, suggesting that as well as for the bacteria-phytoplankton uptake system for the NH4+, the protozoan activity itself seems also to be regulated through Km-Vmax ingestion system for particulate foods. Some experimental results suggested that zooflagellates and small polyhymenophorean oligotrichous ciliates both seem to exhibit High Km-High Vmax ingestion behaviour for bacteria and zooflagellates, respectively. This situation involves an effective ingestion of both bacteria and zooflagellates at vicinities of phytoplankton cells, ultimately leading to an important liberation of NH4+ in that “hot-spots”. On the other hand, there are large polyhymenophorean, oligotrichous, ciliates such as large Oligotrichina and Tintinnina that exhibit low Km-either Low or high Vmax (high flow, Polyhymenophorean, that feed on both zooflagellates and phytoplankton cells outsite and inside of “hot-spots” as well as on whole “hot-spots” themselves widely dispersed in space.
These large ciliates appear thus as major pathway between microbial as well as primary production and higher trophic level at open ocean oligotrophic situations. Indeed, metazoans such as copepods show both preference and high clearance rates on large ciliates relative to small ciliates, further supporting the idea that link to higher trophic levels is through large ciliates which can exist between “hot-spots” patches.
Pushing further the speculation, we could imagine that whole ocean ecosystem is organized in series of nesting boxes (like Russian dolls) of “patches” and “inter-patches” environments inhabited by communities with high Km-high Vmax, and low Km-low Vmax, respectively.
Further Reading
- Ammerman JW, Azam F (1985) Science 227: 1338-1340.
- Azam, F (1998) Science 280: 694-696
- Azam F, Ammerman JW (1984) In Flow of energy and materials in marine ecosystems (ed Fasham MJR) 345-360 (Plenum).
- Azam F, Cho BC (1987) In Ecology of microbial communities (eds) 261-281 (Cambridge University Press).
- Azam F, Smith DC (1991) In Particle analysis in oceanography. (ed Demers S) 213-236 (NATO Series, G27. Springer).
- Azam F, Fenchel T, Field JG, Fray JS, Meyer-Reil LA, Thingstad F (1983) Mar Ecol Prog Ser 10: 257-263.
- Bratback G, Thingstad TF (1985) Mar Ecol Prog Ser 25: 23-30.
- Caron DA, Goldman JC (1990) In Ecology of marine protozoa (ed capriulo GM) 283-306 (Oxford University Press).
- Dolan J (1991) Estuarine, Coastal and Shelf Science 33: 137-152.
- Ducklow HW, Purdie DA, Williams LeBPJ, davis JM (1986) Science 232: 865-867.
- Fenclel T (1980a) Limnol Oceanogr 25: 735-740.
- Fenchel T (1980b) Arch. Protistenk 123: 239-260.
- Ferrier C, Rassoulzadegan F (1991) Limnol Oceanogr 36: 657-669.
- Hagström A, Azam F, Andersson A, Wikner J, Rassoulzadegan F (1988) Mar Ecol Progr Ser 49: 171-178.
- Kirchman, DL (ed) (2000) Microbial Ecology of the Oceans. (Wiley-Liss New York)
- Pomeroy LR (1974) BioScience 24: 499-504.
- Rassoulzadegan F (1990) Zoological science 7 (S): 189-196.
- Rassoulzadegan F (1993) In Trends in Microbial Ecology (eds Guerrero R. & Pedros-Alio C) 435-439 (Spanish Society for Microbiology).
- Rassoulzadegan F, Sheldon RW (1986) Limnol Oceanogr 31: 1010-1021.
- Rivier A, Browlee, DC, Sheldon RW, Rassoulzadegan F (1985) mar Micro Food Webs 1: 36-51.
- Sherr EB, Sherr BF, Fallon RD, Newell SY (1986) Limnol Oceanogr 31: 177-183.
- Stoecker DK, Capuzzo JM (1990) J Plankton Res 12: 891-908.
- Tiselius P (1989) mar Ecol Prog ser 56: 49-56.
- Wiadnyana NN, Rassoulzadegan F (1989) Mar Ecol Prog Ser 53: 37-45.