Isomerization in petroleum refining
There are two distinct isomerization processes, butane (C4) and pentane/hexane (C5/C6) Isomerization is a process in petroleum refining that converts n-butane, n-pentane and n-hexane into their respective isoparaffins of substantially higher octane number. The straight-chain paraffins are converted to their branched-chain counterparts whose component atoms are the same but are arranged in a different geometric structure. Isomerization is important for the conversion of n-butane into isobutane, to provide additional feedstock for alkylation units, and the conversion of normal pentanes and hexanes into higher branched isomers for gasoline blending. Isomerization is similar to catalytic reforming in that the hydrocarbon molecules are rearranged, but unlike catalytic reforming, isomerization just converts normal paraffins to isoparaffins.
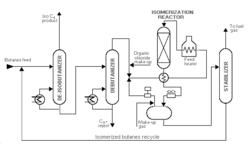
Butane isomerization produces feedstock for alkylation. Aluminum chloride catalyst plus hydrogen chloride are universally used for the low-temperature processes. Platinum or another metal catalyst is used for the higher-temperature processes. In a typical low-temperature process, the feed to the isomerization plant is n-butane or mixed butanes mixed with hydrogen (to inhibit olefin formation) and passed to the reactor at 230°-340° F and 200-300 psi. Hydrogen is flashed off in a high-pressure separator and the hydrogen chloride removed in a stripper column. The resultant butane mixture is sent to a fractionator (deisobutanizer) to separate n-butane from the isobutane product.
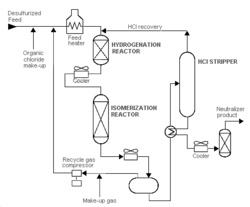
Pentane/hexane isomerization increases the octane number of the light gasoline components n-pentane and n-hexane, which are found in abundance in straight-run gasoline. In a typical C5/C6 isomerization process, dried and desulfurized feedstock is mixed with a small amount of organic chloride and recycled hydrogen, and then heated to reactor temperature. It is then passed over supported-metal catalyst in the first reactor where benzene and olefins are hydrogenated. The feed next goes to the isomerization reactor where the paraffins are catalytically isomerized to isoparaffins. The reactor effluent is then cooled and subsequently separated in the product separator into two streams: a liquid product (isomerate) and a recycle hydrogen-gas stream. The isomerate is washed (caustic and water), acid stripped, and stabilized before going to storage.
| Disclaimer: This article is taken wholly from, or contains information that was originally published by, the Occupational Safety & Health Administration (OSHA). Topic editors and authors for the Encyclopedia of Earth may have edited its content or added new information. The use of information from the Occupational Safety & Health Administration (OSHA) should not be construed as support for or endorsement by that organization for any new information added by EoE personnel, or for any editing of the original content. |