Converting Cellulose to Ethanol
| Topics: |
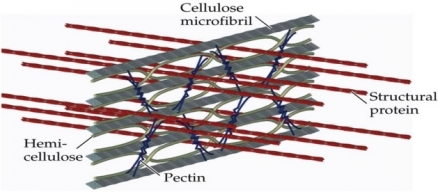
Structural integrity of plants depends on cellulose, a polymer that, like starch, is composed of glucose subunits. The glucose subunits in cellulose alternate direction, however, and this configuration promotes the formation of strong chemical bonds (hydrogen bonds) between adjacent cellulose chains. Plant cell walls contain dozens of cellulose chains, packed together into microfibrils; these microfibrils intertwine with hemicelluloses, pectins, proteins, and lignins. This complex construction gives cell walls the strength to withstand pressures that exceed 1.5 MPa (218 psi) and to resist pathogen invasion and herbivore attack. Unfortunately, this strength exposes the weakness of many mitigation strategies involving biofuels: Cell walls are highly recalcitrant to most physical and chemical treatments that would convert them into ethanol.
The first step in processing ethanol from cellulose is the liberation of cellulose and hemicellulose from cell walls. The paper manufacturing industry has centuries of experience in cellulose processing. The paper industry grinds or steam explodes plant material and then subjects it to solutions of hot, concentrated base (sodium hydroxide) or acid (sulfuric acid). Unfortunately, this process is energy intensive, expending about 30 MJ per kg of paper, more than the energy value of the paper [1], and generates a range of products that not only inhibit subsequent steps in converting cellulose into ethanol, but are potentially harmful to the environment. Paper manufacturing is only the twelfth largest industry in the United States according to product value [2], but is the fifth largest polluter after metal mining, electric utilities, chemicals, and metal smelting. [3] Therefore, processors of biofuels seek improved methods of releasing cellulose and hemicellulose from cell walls.
The next step is the decomposition of cellulose and hemicellulose into sugars via hydrolysis. Given the stability and complexity of these compounds, no single enzymatic or chemical treatment achieves adequate yields or rates. The long periods required for hydrolysis increase the possibility of contamination by microorganisms that consume sugars without producing anything of human value. Extensive research efforts are underway to develop a fast, efficient method to hydrolyze cellulose and hemicellulose into sugars.
The step that follows hydrolysis is fermentation, in which microbes convert sugars into ethanol. Yeast are highly effective at converting hexoses (six-carbon sugars such as glucose and fructose), which are produced from sugarcane or during hydrolysis of maize starch. They are less effective at converting pentoses (fivecarbon sugars such as xylose and arabinose), which are generated during the hydrolysis of hemicellulose. For instance, about 30% of the sugars produced during hydrolysis of maize stover (mature stalks minus the ears) is xylose, which yeast fermentation cannot efficiently convert to ethanol. Genetic engineering of bacteria (e.g., Zymomonas mobilis and Escherichia coli) as well as fungi (e.g., Saccharomyces cerevisiae and Pichia stipitis) for efficient ethanol production from pentoses is an active area of research. [4]
In summary, despite the promise of cellulosic ethanol, most of the steps in processing are still experimental, and the costs are not yet competitive with other energy sources.
[1] Patzek, T. W. (2006) The Real Biofuel Cycles, Online supporting material for Science 312: 1747 (2006), Berkeley, CA, http://petroleum.berkeley.edu/patzek/BiofuelQA/Materials/RealFuelCycles-Web.pdf.
[2] Bureau of Economic Analysis (2008) Gross Output by Industry. U.S. Department of Commerce, Gross Domestic Product by Industry Accounts, http://www.bea.gov/industry/gpotables/gpo_action.cfm?anon=70583&table_id=22079&format_, accessed May 20, 2008.
[3] U.S. Environmental Protection Agency (2007b) TRI Explorer. http://www.epa.gov/triexplorer/industry.htm, accessed Sept. 11, 2007.
[4] Jeffries, T. W. and Y. S. Jin (2004) Metabolic engineering for improved fermentation of pentoses by yeasts. Applied Microbiology and Biotechnology 63:495-509.