Carbon Storage in the Deep Ocean
Carbon Storage in the Deep Ocean
| Topics: |
Earth’s oceans are vast, covering over 70% of the planet’s surface at an average depth of 3800 meters. Injecting CO2 into the deep ocean would isolate it from the atmosphere for several centuries and halve peak atmospheric concentrations. Eventually, after about 2 millennia, CO2 concentrations in the atmosphere and oceans would come back into equilibrium. Therefore, oceans can offer long-term, but not permanent, storage.
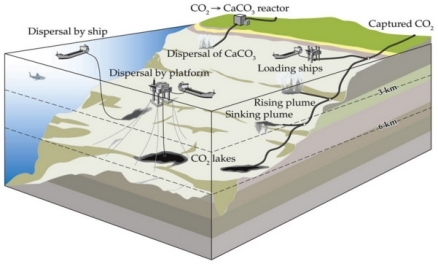
Several mitigation strategies envision that electric power plants would pump captured CO2 down pipelines into the ocean depths. Alternatively, tanker ships carrying CO2 would release it at depth or would carry the CO2 to platforms that release it at depth. Finally, some plants would convert CO2 into CaCO3 and dissolve it in seawater for release at depth.
The fate of CO2 in water is complex, both in its dissociation into H2CO3, HCO3 –, and CO3 2– and in its transitions among gas, liquid, and solid phases. In oceans, as depth increases, hydrostatic pressure increases and temperature drops. Injected CO2 remains a gas until about 400 m depth, at which point it becomes a supercritical fluid (its density varies continuously with pressure, without a distinct phase change).
For reasons that are not well understood, a portion of the CO2 at these depths forms hydrates. In a hydrate, a solid, crystalline cage of water molecules surrounds each CO2 molecule (CO2·6H2O). Hydrates of CO2, denser than seawater, tend to sink in the water column, whereas liquid CO2, less dense than seawater at depths between 400 m and 2700 m, tends to rise. Below 3000 m, liquid CO2 becomes denser than seawater and sinks. Injection of CO2 into deep water, therefore, may establish CO2 lakes in depressions on the sea bottom. Such lakes would delay the dispersion of CO2 into the surrounding environment.
Large-scale injection of captured CO2 into the oceans would influence sea life. High CO2 concentrations themselves can interfere with aerobic respiration. Moreover, liquid CO2 behaves as an organic solvent that may prove toxic to certain organisms. Finally, dissolution of CO2 into its various bicarbonate and carbonate forms would acidify the surrounding water, and sea life would respond to these pH changes. Nonetheless, we have such limited knowledge about life in deep oceans that we cannot anticipate all of the consequences of massive CO2 injections. [1]
Other carbon dioxide storage methods include converting CO2 into mineral carbonates and pumping CO2 into the deep sea.
[1] Nealson, K. (2006) Lakes of liquid CO2 in the deep sea. Proceedings of the National Academy of Sciences of the United States of America 103:13903-13904.
This is an excerpt from the book Global Climate Change: Convergence of Disciplines by Dr. Arnold J. Bloom and taken from UCVerse of the University of California.
©2010 Sinauer Associates and UC Regents
0 Comments