Health and safety aspects of in-situ burning of oil
Contents
Background
In-situ burning is the combustion of a spill (Oil spill) product at the site of the spill (Oil spill) (in situ is Latin for “in place”.) In-situ burning of oil may offer a logistically simple, rapid, inexpensive, and relatively safe means for reducing the net environmental impact of an oil spill. Because a large portion of the oil is converted to gaseous combustion products, the need for collection, storage, transport, and disposal of recovered material can be substantially reduced.
Required Conditions
To burn oil on water, four major conditions must be met:
- The oil layer has to be thick enough to support combustion. Oil layers thinner than 1 to 2 mm lose too much heat to the water and cannot support combustion.
- The ignition devices used must be hot enough and last long enough to ignite the oil.
- The water-in-oil emulsion may not contain more than 30 to 50 percent water to ignite and support combustion.
- To use currently available fire resistant booms, environmental conditions must be favorable: wind speed should be below 20 knots, and wave height should be below three feet.
Burning Technique
An in-situ burning technique likely to be used would employ towing boats and fireresistant booms to contain the spilled oil and keep it from spreading. The boom, attached to the boats by towing lines, would be pulled by the boats to form a U shape. The open end of the U is maneuvered through the oil slick, and a "boomful" of oil is collected. The boom is towed away from the main slick and the oil is ignited. During the burning, the boom is pulled so that it slowly advances ahead of the current to ensure that the oil is concentrated at the back end of the boom and maintains maximum thickness. After the oil is burned, the process may be repeated for as long as in-situ burning is feasible.
Burning Efficiency
In-situ burning has been studied under controlled conditions in laboratories and in field tests, and recently under realistic conditions in an experiment off the coast of Newfoundland, Canada. This experience indicated that in-situ burning can be an effective oil-removing technique, removing 50 to 99 percent of the oil collected in the boom. In addition, a field "real" burn conducted in the first days of the Exxon Valdez spill in Prince William Sound, Alaska, resulted in the burning of 15,000 to 30,000 gallons of Prudhoe Bay crude oil at an estimated efficiency of 98 percent or better (Allen 1990).
Plume Behavior
The heat generated by the burning oil in the boom (1800 °F were measured at the top of the boom at the Newfoundland burn) will cause the smoke to rise several hundred to several thousand feet, and at the same time be carried away by the prevailing [[wind]s]. In areas having well-developed sea-breeze systems, plume fumigation and recycling may draw the smoke toward the surface. It is expected that the plume will be high enough to stay out of the sea-breeze/land-breeze circulation cell. The smoke plume at the in-situ burning conducted off Newfoundland and at marsh and brush burns leveled off at several hundred feet, and then lofted slowly over distance. The upper boundary of the plume often extends to an altitude of several thousand feet. The main plume may also split into two or more smaller plumes, each heading in a somewhat different direction.
Human Health Consideration
The possible health hazards of in-situ burning to nearby response personnel conducting the burn will be different from those for the general public at a substantial distance away.
Response personnel: Response personnel working close to the burn may be exposed to levels of gases and particulates that would require them to use personal protective equipment. They should receive the training required to conduct the operation safely. Monitoring of the responders' work environment should be conducted as needed. Occupational standards such as OSHA's Permissible Exposure Limits (PEL) are applicable to this group of typically healthy adults.
General public: Based on data from the Newfoundland Offshore Burn Experiment (NOBE) (Ferek 1994; Fingas et al. 1994; Bowes 1994) and previous burns (Fingas et al. 1993; Booher 1992; Evans et al. 1992), particulate concentrations in the smoke plume remain the only agent of concern past a mile or two downwind, the gases created in the burn having dissipated to levels close to background. Public exposure to smoke particulate from the burn is not expected to occur unless the smoke plume goes down to ground level. Since the general public may include sensitive individuals such as the very young and very old, pregnant women and people with pulmonary or cardiovascular diseases, this population's tolerance to particulates may be significantly lower than that of the responders. Protecting the general public may be achieved by minimizing exposure and conducting the burn only when conditions are favorable and exposure to particulates from the burn is below the level of concern. There is little data concerning the effect of particulates from in-situ burning of oil on humans. Based on chemical analysis of soot particulates and their physical behavior, the hazard is expected to be similar to that of better known particulates emissions now regulated by the National Ambient Air Quality Standard (NAAQS).
Determining the level of concern for exposure to particulates is not simple. The existing NAAQS of 50 µg/m3 annual mean and 150 µg/m3 averaged over 24 hours is designed for continuous sources such as industry and motor vehicle emissions. In-situ burning is likely to occur over a short period of time: hours, perhaps a day or two. Is the existing PM-10 standard well suited for in-situ burning? This issue definitely warrants further consideration.
Toxic Components of the Smoke Plume
The smoke emitted from oil combustion contains gases and particulates that may have toxic effects on our bodies, much like exhaust emissions from motor vehicles or smoke from wood stoves. The health risk will depend on the actual exposure to these agents.
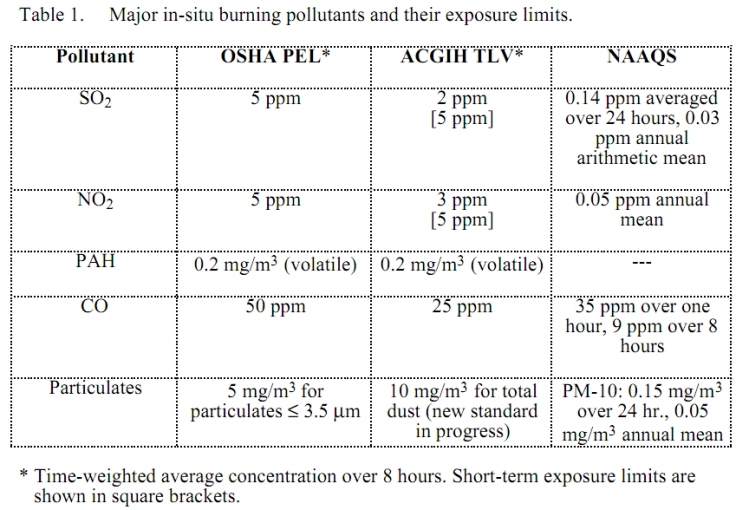
Most of the oil in in-situ burning will be converted to carbon dioxide and water. Particulates, mostly soot, comprise ten to fifteen percent of the smoke plume. Small amounts of toxic gases are emitted as well. These include sulfur dioxide, nitrogen dioxide, and carbon monoxide. In addition, small amounts of polynuclear aromatic hydrocarbons (PAHs) are emitted from the fire, mostly as residues attached to the particulates. These combustion by-products are discussed below, and their NAAQS and occupational exposure limits are shown in the following table.
Sulfur dioxide (SO2) is a gas formed when sulfur in the oil or hydrogen sulfide (Health effects of hydrogen sulfide) coming out of a well oxidize during the combustion process. This gas is toxic and irritates the eyes and respiratory tract by forming sulfuric acid (Health effects of sulfur trioxide and sulfuric acid) on these moist surfaces (Amdur 1986). The concentration of SO2 in the smoke plume depends on the sulfur content of the oil. Average SO2 levels measured in experimental burns have been below 2 ppm in the plume 100-200 meters downwind of the burn (Fingas et al. 1993). Several miles downwind, sulfur dioxide from in-situ burning is expected to be much below the level of concern for the general population.
Nitrogen dioxide (NO2) is another gaseous by-product of oil combustion. Like SO2, it is reactive, toxic, and a strong irritant to the eyes and respiratory tract. NO2 is less soluble than SO2 and therefore may reach the deep portions of the lungs (the critical gas exchange area of the lungs) so that even low concentrations may cause pulmonary edema, which may be delayed (Amdur 1986).
Sampling results to date indicate that the concentration of nitrogen dioxide in the plume several miles downwind of the burn does not exceed several parts per billion (Ferek 1994). Therefore, it is not expected to pose a threat to the general public several miles downwind of the burn.
Polynuclear aromatic hydrocarbons (PAHs) are a group of hydrocarbons characterized by multiple benzene rings attached together. These compounds have very low vapor pressures and are not very flammable (compared to other compounds found in [[crude oil]s]). PAHs are found in the unburned oil as well as the smoke plume. Some PAHs are known or suspected carcinogens. Target organs may include the skin (from chronic skin contact with oils) or the lungs from inhalation of aerosol. Based on data from NOBE and previous burns, most PAHs are burned in the fire, and their concentration in the oil residue is higher than in the smoke plume (Fingas et al. 1994).
PAHs were found in barely detectable concentrations in the smoke from the Kuwaiti oil fires (Campagna and Humphrey 1992). Low levels of PAHs were also detected in experimental oil burns, (Mass Selective Detector, analytical sensitivity 0.01 µg/m3 air) with levels in the plume less than 0.01 ppm (Fingas et al. 1994). Considering the low level of PAHs detected so far, it is felt that they present only a small exposure hazard.
Carbon monoxide (CO) is a common by-product of incomplete combustion. The toxicity of CO is acute and stems from its high affinity to the hemoglobin molecule in red blood cells. CO will chemically displace oxygen from the blood and cause oxygen deprivation in the cells of the body. In experimental burns the average level of CO in the smoke plume over the duration of the burns (15 to 30 minutes) was found to be 1 to 5 ppm 150 meters downwind of the burns (Fingas et al. 1993).
in the smoke plume are considered by most health professionals to be the main combustion product to investigate and monitor. Therefore, particulates will be discussed in more detail.
Particulates are small pieces of solid materials (dusts, soot, fumes) or liquid material (mists, fogs, sprays) that remain suspended in the air long enough to be inhaled. During in-situ burning elemental carbon (soot) and hydrocarbons are emitted. Since these particulates absorb light to a high degree, the smoke plume is usually black.
Particulate concentration is measured in several ways. A relatively accurate method involves sampling with an air pump that draws air through a filter. Depending on pore size, the filter may collect more than 99.9 percent of the particulates in the air. Real-time instruments that can measure particulate concentration at the time of measurement are also available; some are quite sensitive and accurate. They must be calibrated to the particulates of concern, and may be affected by other [[aerosol]s] such as water vapor.
Since 10 micrometers (µm) in diameter is the size below which particulates may be inhaled and become a burden on the respiratory system, scientists divide the particulate mass into “total” particulates, which include any size measurable, and “PM-10,” which is the fraction of particulates smaller than 10 µm in diameter. The combined effect of the anatomical structure of the respiratory system and physical behavior of particles cause particles greater than 10 µm in diameter to be deposited and removed from the air stream at the nose and upper portion of the respiratory tract. Particles 5 to 10 µm in diameter would be deposited along the elaborate air conducting tube system, the bronchi. Only particles smaller than 5 µm will actually be deposited in the deeper portion of the lungs, in the alveoli, which are the little sacs where gas exchange takes place. The median size of the particulates reaching the alveoli is approximately 0.5 µm in diameter, meaning that half the number of particulates will be smaller than 0.5 µm, and half will be larger. Only 2 percent of the particulates found in the alveoli would be larger than 3 µm (Wright 1978).
Particulate size also plays a crucial role in determining how long they will be suspended in the air. Larger particulates (tens of µm in diameter) would precipitate rather quickly close to the burning site. Smaller particulates (ranging from a fraction of a µm to several µm in diameter) would stay suspended in the air for a long time and be carried over long distances by the prevailing [[wind]s]. Particulates small enough to be inhaled (PM-10) are also the ones to remain suspended. A practical implication is that if those particulates do not descend to ground level (where people are) they will not threaten the population downwind.
For most people, exposure to inert respirable particulates may become a problem at high concentrations (several milligrams of particulates per cubic meter of air.) However, sensitive individuals may develop respiratory problem at levels much lower than that. Several recent studies (Schwartz 1992; Pope et al. 1992; Dockery et al. 1992) suggest that there is a correlation between particulate concentration in the air and daily mortality. These studies used measurements of air pollution and matched them to mortality and morbidity data in several cites in the United States: Philadelphia; Detroit; Provo, Utah; and Birmingham, Alabama. Higher levels of PM-10 were associated with an increase in daily morbidity and mortality, especially among older people and people with allergies, respiratory problems, or cardiovascular diseases. An increase of 100 µg/m3 of the measured daily particulate level was associated with six percent increase in mortality (Schwartz 1992). The biological mechanism has not been determined, but the possibility of such a correlation should dictate that in-situ burning be conducted only when it does not pose a hazard to human health, and exposure to particulates should not exceed the applicable federal or state standard.
Sampling conducted so far indicates that the population downwind and even response personnel will be exposed to very low levels of gases and particulates. In the recent experimental in-situ burn off the coast of Newfoundland, many participants were tagged with sampling badges to assess their exposure to volatile organic compounds (VOCs.) Initial analysis of those badges indicates that exposures in most cases were below the level of detection (LOD = 0.001 mg per sample.) The few detected VOC "hits" could be traced to fuel and solvents on the vessels rather than VOCs from the spilled or burning oil (Bowes 1994). Similarly, the level of respirable particulates (PM-10) was monitored by a University of Washington research aircraft. The general trend showed variable concentration of PM-10 in the smoke plume. While at several spots the concentration of particulates exceeded 150 µg/m3 even 10 miles downwind of the burn, other places in the plume had particulate concentrations lower than 150 µg/m3. The most remarkable finding is that PM-10 concentration beneath the plume, 150-200 feet above the surface, did not exceed background levels of 30 to 40 µg/m3 (Ferek personal communication 1994 ). These data agree well with previous measurements done in test burns in Mobile Bay, Alabama.
Safety Hazards
Safety hazards for in-situ burning operations should be similar to those of ordinary skimming at sea, with the added hazards related to the combustion process:
- In-situ burning is a process that involves the intentional setting of a fire. Great care must be taken so that this fire is controlled at all times.
- Ignition of the oil slick, especially by aerial ignition methods (such as the helitorch), must be well coordinated with neighboring vessels and be carefully executed. Weather and water conditions should be kept in mind, and proper safety distances should be kept at all times.
- In-situ burning at sea will involve several vessels working relatively close to each other, perhaps at night or in other poor-visibility conditions. Such conditions are hazardous by nature and require a great degree of practice, competence, and coordination.
- Response personnel must receive the appropriate safety training. Training should include proper use of personal protective equipment, respirator training and fit testing, heat stress considerations, first aid, small boat safety, and any training required to better prepare them to perform their job safely.
Safety hazards are substantial and should be given due attention. Usually they pose a much greater risk to the responders than the previously discussed chemical exposure.
In-situ burning will require only a fraction of the people needed to clean the same amount of oil if it impacts the beaches. In addition, personnel conducting the burn are expected to be well trained and monitored and, hopefully, have a low accident rate. In-situ burning, by minimizing the amounts of oil impacting the beaches, may prevent the illnesses and injuries that are often associated with beach cleanup operations.
Environmental Considerations
While the main purpose of this brief review is to present the major human health and safety considerations of in-situ burning, mentioning the greater health aspects that affect our environment and, ultimately, our quality of life is definitely warranted. We will touch on a few points only. These points include the feasibility of burning the oil as opposed to leaving it to evaporate, waste generation, and possible effects on exposed wildlife.
Burning vs. Evaporation
A point to keep in mind is that leaving the oil in place will have a deleterious effect on air quality. Spilled oil left untreated would evaporate at a rate that depends on the type of oil, time elapsed from release, wind, Waves, and water and air temperatures. The amount evaporated is substantial. For example, 32 percent of spilled Alberta Sweet crude would evaporate after 24 hours in 80 degree water, and after five days 42 percent would have evaporated. This evaporation pattern, similar in other oil types, emphasizes the need for quick action if in-situ burning is selected as the response tool.
The decision whether to burn or not to burn involves a tradeoff: burning the oil would reduce or eliminate the [[environmental impact] of the oil slick] and convert most of the oil to carbon dioxide and water. Burning, however, would generate particulates and cause air pollution. Not burning the oil would enable the slick to spread over a large area and impact the environment. Particulates would not be produced, but up to 50 percent of the oil would evaporate, causing a different kind of air pollution.
Waste Generation
Mechanical cleanup of oil spills generates large amounts of waste. It was estimated that 350 miles of sorbent boom was used during the first summer of the Exxon Valdez cleanup (Ferriere 1993), more than 25,000 tons of sorbent material of all kinds was sent to landfills, and oily water twice the amount of the oil spilled (from skimming a fraction of the oil) had to be treated (Fahys 1990). Enough energy was used that summer to support the energy needs of 11,000 people, power 1,300 boats of all sizes, and provide hot water equal to the need of a city of 500,000 people (Ferriere 1993).
In-situ burning of oil is going to generate waste. Even the most efficient burning will leave a taffy-like residue that will have to be collected and treated or disposed of. Burning the oil at sea will not be as efficient as burning it in engines, furnaces, or power plants, and will generate a substantial amount of particulates. However, by minimizing the solid and liquid waste generated by beach cleanup, and by reducing the energy required to support the response operation, burning even some of the oil at sea is likely to reduce the overall waste generation of a spill (Oil spill).
Effects on Birds and Mammals
Based on our limited experience, birds and mammals are more capable of handling the risk of a local fire and temporary smoke plume than of handling the risk posed by a spreading oil slick. Birds flying in the plume can become disoriented, and could suffer toxic effects. This risk, however, is minimal when compared to oil coating and ingestion, the result of birds' exposure to the oil slick.
The effect of in-situ burning on mammals is yet to be seen. It is not likely that sea mammals will be attracted to the fire, and the effect of smoke on marine mammals is likely to be minimal. Mammals, on the other hand, are adversely affected by oil ingestion and oil coating of their fur. Therefore, reducing the spill size by burning the spilled oil can reduce the overall hazard to mammals.
Once coated by oil, neither birds nor mammals have responded well to rehabilitation efforts, and although much has been learned and rehabilitation methods have greatly improved, the success rate of wildlife rehabilitation has been moderate at best.
Conclusions
In-situ burning of oil may provide an efficient and rapid method of oil spill response, providing that the requirements to carry on the response are met. Burning the oil on the water generates a large amount of smoke, which contains particulates and toxic gases. Among those, particulates seem to be the major agent of concern, as their concentration in the center of the plume remains above the level of concern for the general population for several miles downwind. It was found, however, that particulates concentration under the plume does not significantly exceeds background levels. Protection of response personnel can be achieved by adequate training and personal protective equipment. The general public can be protected by establishing burning guidelines that will prevent the burn from becoming a health hazard to the public.
When compared to conventional response methods and to beach cleanup, in-situ burning can reduce the number of people required to clean the beaches, and reduce the injuries associated with this hazardous work. By eliminating the oil at the source of the spill (Oil spill), contact with oil by marine birds and mammals can be reduced. Burning the oil to minimize beach impact will reduce the waste generated by conventional beach cleanup. While generating substantial amounts of combustion by-products, mostly carbon dioxide, water, and particulates, in-situ burning reduces the amount of VOCs evaporating from the spilled oil.
Since in-situ burning of oil has the potential to reduce the destructive impact of [[oil spill]s], and since the risk it poses to the responders and to the population downwind are, under most circumstances, acceptable, it should be one of the response options available to combat future [[oil spill]s].
References
- Allen, A.A. 1990. Contained controlled burning of spilled oil during the Exxon Valdez oil spill. Proceedings of the Thirteenth Arctic and Marine Oil Spill Program Technical Seminar, June 6-8, 1990, Edmonton, Alberta, pp. 305-313. Amdur, M.O. 1986. Air pollutants. In C.D. Klaassen, M.O.
- Amdur, and J. Doull, eds., Casarett and Doull's Toxicology: The Basic Science of Poisons. New York: Macmillan Publishing Co., pp. 801-824.
- Booher, L.E., Exxon Corporation USA, Baton Rouge, Louisiana. Personal communication, October 1992.
- Bowes, S., Exxon Biomedical. East Millstone, New Jersey. Personal communication, February and May, 1994.
- Campagna, P.R. and A. Humphrey. 1992 Air sampling and monitoring at the Kuwait oil well fires. Proceedings of the Fifteenth Arctic and Maine Oil Spill Program Technical Seminar, June 10-12, 1992, Edmonton, Alberta, pp. 575-592.
- Dockery, D.W., J. Schwartz, and J. D. Spengler. 1992. Air pollution and daily mortality: associations with particulates and acid aerosols. Environmental Research 59: 362-373.
- Evans, D.D., W.D. Walton, H.R. Baum, K.A. Notarianni, J.R. Lawson, H.C. Tang, K.R. Keydel, R.G. Rehm, D. Madrzykowski, R.H. Zile, H. Koseki, and E.J. Tennyson. 1992. In-situ burning of oil spills: Mesoscale experiments. Proceedings of the Fifteenth Arctic and Marine Oil Spill Program Technical Seminar, June 10-12, 1992, Edmonton, Alberta, pp. 593-657.
- Experimental Burn Committee, 1993. NOBE Facts. Volume 5, September 1993. Ottawa, Ontario: Newfoundland Burn Experiment Committee.
- Fahys, J. 1990. Exxon officials rate Valdez waste management plan a success. HAZMAT World, February 1990, pp 28-30.
- Ferek, R. 1994. Personal communication, March 1994.
- Ferriere, D. 1993. Waste minimization concepts applied to oil spill response. Proceedings of the International Oil Spill Conference, March 29-April 1 1993, Tampa, Florida, pp 111-1115.
- Fingas, M. F., K. Li, F. Ackerman, P. R. Campagna, R. D. Turpin, S. J. Getty, M. F. Soleki, M. J. Trespalacios, J. Pare, M. C. Bissonnette, and E. J. Tennyson. 1993. Emissions from mesoscale in-situ oil fires: the Mobile 1991 and 1992 tests. Proceedings of the Sixteenth Arctic and Marine Oil Spill Program Technical Seminar, June 7-9, 1993, Calgary, Alberta, pp. 749-823.
- Fingas, M.F., F. Ackerman, K. Li, P. Lambert, Z. Wang, M. C. Bissonnette, P.R. Campagna, P. Boileau, N. Laroche, P. Jokuty, R. Nelson, R. Turpin, M.J. Trespalacios, G. Halley, J. Belanger, J. Pare, N. Vanderkooy, E. Tennyson, D. Aurand, and R. Hiltabrand. 1994. The Newfoundland offshore burn experiment - NOBE preliminary results of emissions measurement. Proceedings of the Seventeenth Arctic and Marine Oil Spill Program Technical Seminar, June 8-10, 1994, Vancouver, British Columbia, pp. 1099-1164. 9
- Pope, C.A. III, J. Schwartz, and M.R. Ransom. 1992. Daily mortality and PM-10 pollution in Utah valley. Archives of Environmental Health 47: 211-217
- Schwartz, J., 1992. Particulate air pollution and daily mortality: a synthesis. Public Health Review 19: 39-60
- Wright, G.R. 1978. The pulmonary effects of inhaled inorganic dust. G.D. Clayton and F.E. Clayton, eds., Patty's Industrial Hygiene and Toxicology, Volume 1: General. New York: John Wiley and Sons. pp. 165-202.