Interaction of chemicals in the body
Humans can normally be exposed to several chemicals, such as environmental pollutants in food, water, and air and therapeutic agents, rather than to an individual chemical. Examples are: hospital patients on the average receive 6 drugs dailyhome influenza treatment consists of aspirin, antihistamines, antibiotics, and cough syrup taken simultaneouslydrinking water and food may contain small amounts of organic (pesticides (Interaction of chemicals in the body) , solvents, PCBs, PCDDs, etc.) and inorganic (heavy metals) chemicals * air often contains mixtures of hundreds of chemicals such as urban and industrial combustion products, cigarette smoke, etc. * gasoline vapors include several hydrocarbons and additives * toxicity of a specific chemical is tested by exposing experimental animals to only one chemical at a time. Toxicity testing of mixtures is usually difficult because it is impossible to predict the possible combinations of chemicals that will be present in multiple-chemical exposures.
Xenobiotics (foreign chemicals) administered simultaneously may act independently of each other. However, in many cases, the presence of one chemical may drastically affect the response to another chemical. The effectiveness or toxicity of a mixture of chemicals may be less or more than would be predicted from the known effects of each individual chemical. The effect that one chemical has on the therapeutic/toxic effect of another chemical is known as an interaction.
Contents
Types of Interactions
There are four basic types of interactions. Each is based on the expected effects caused by the individual chemicals. The types of interactions are:
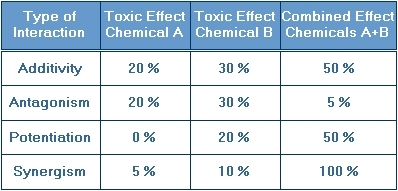
This table quantitatively illustrates the percent of the population affected by individual exposure to chemical A and chemical B as well as exposure to the combination of chemical A and chemical B. It also gives the specific type of interaction:
The interactions described can be categorized by their chemical or biological mechanisms as follows:
- chemical interaction/s between administered chemicals
- modifications in absorption, metabolism, storage, or excretion of one chemical by the other
- reactions at binding sites and receptors
- due to physiological changes caused by other chemicals
Additivity
Additivity is the most common type of drug interaction. Examples of chemical or drug additivity reactions are:
- Two central nervous system (CNS) depressants taken at the same time, a tranquilizer and alcohol, often cause depression equal to the sum of that caused by each drug.
- Organophosphate insecticides interfere with nerve conduction. The toxicity of the combination of two organophosphate insecticides is equal to the sum of the toxicity of both.
- Chlorinated insecticides and halogenated solvents both produce liver toxicity. The hepatotoxicity of an insecticide formulation containing both can be equivalent to the sum of the hepatotoxicity of both.
Potentiation
Potentiation occurs when a chemical that does not have a specific toxic effect makes another chemical more toxic. Examples are:
- The hepatotoxicity of carbon tetrachloride is greatly enhanced by the presence of isopropanol. Such exposure may occur in the workplace.
- Normally, warfarin (a widely used anticoagulant/blood thinner in cardiac disease) is bound to plasma albumin so that only 2% of the warfarin is active. Drugs which compete for binding sites on albumin increase the level of free warfarin to 4% causing fatal hemorrhage.
Synergism
Synergism can have serious health effects. With synergism, exposure to a chemical may drastically (more than additive) increase the effect of another chemical. Examples are:
- Exposure to both cigarette smoke and radon and asbestos results in a significantly greater risk of lung cancer than the sum of the risks of each.
- The hepatotoxicity of a combination of ethanol and carbon tetrachloride is much greater than the sum of the hepatotoxicity of each.
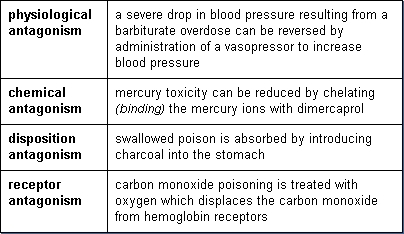
Antagonism
Antagonism is often a desirable effect in drug toxicity and is the basis for most antidotes. Examples include:
Different types of interactions can occur at different target sites for the same combination of two chemicals.
For example, chlorinated insecticides and halogenated solvents can produce liver toxicity with the interaction being additive. However, the same combination of chemicals can produce a different type of interaction on the central nervous system. Chlorinated insecticides stimulate the central nervous system whereas halogenated solvents cause its depression. The net effect of simultaneous exposure is an antagonistic interaction. Liver enzymes oxidize sulfur containing organophosphate insecticides, but antioxidants can inhibit these enzymes and can protect against their neurotoxicity.
| Disclaimer: This article is taken wholly from, or contains information that was originally published by, the National Library of Medicine. Topic editors and authors for the Encyclopedia of Earth may have edited its content or added new information. The use of information from the National Library of Medicine should not be construed as support for or endorsement by that organization for any new information added by EoE personnel, or for any editing of the original content. |