Marine carbonate chemistry
Contents
Introduction
The ocean represents the largest surficial carbon reservoir (~38,000 Pg C) of Earth's carbon cycle. The mean concentration of inorganic carbon in the ocean is about 2.3 mmol kg-1; its residence time is ~200 ka. Ocean and atmosphere exchange carbon in the form of carbon dioxide (CO2). Atmospheric CO2 is therefore strongly coupled to the oceanic reservoir. The total amount of dissolved inorganic carbon in the modern ocean is about sixty times larger than of the pre-anthropogenic atmosphere.
Dissolved Carbon Dioxide
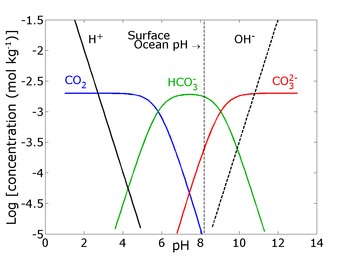
Dissolved
in seawater occurs mainly in three inorganic forms (see Figure): free aqueous carbon dioxide (CO2(aq)), bicarbonate (HCO3-), and carbonate ions (CO32-).
A minor form is true carbonic acid (H2CO3) whose concentration is less than 0.3% of [CO2(aq)]. The sum of [CO2(aq)] and [H2CO3] is denoted as [CO2]. The majority of dissolved inorganic carbon in the ocean is in the form of HCO3- (>85%). Gaseous carbon dioxide (CO2(g)), and [CO2] are related by Henry's law in thermodynamic equilibrium:
CO2(g) = CO2; K0 (1)
where K0 is the solubility coefficient of CO2 in seawater. The concentration of dissolved CO2 and the fugacity of gaseous CO2, fCO2, then obey the equation [CO2] = K0 × fCO2. The fugacity is practically equal to the partial pressure, pCO2 (within ~1%). The dissolved carbonate species are related by:
CO2 + H2O = HCO3- + H+; K*1 (2)
HCO3- = CO32- + H+; K*2 (3)
The pK*'s (= −log(K*)) of the stoichiometric dissociation constants of carbonic acid in seawater are pK*1 = 5.94 and pK*2 = 9.13 at temperature T=15°C, salinity S=35, and surface pressure P=1 atm on the total pH scale. At typical surface seawater pH of 8.2, the speciation between [CO2], [HCO3-], and [CO32-] hence is 0.5%, 89%, and 10.5%, showing that most of the dissolved CO2 is in the form of HCO3- and not CO2 (see Figure). The sum of the dissolved carbonate species is denoted as total dissolved inorganic carbon (DIC ≡ ΣCO2 ≡ TCO2 ≡ CT):
TCO2 = [CO2] + [HCO3-] + [CO32-] . (4)
Another critical parameter to describe the marine carbonate system is the total alkalinity (TA), which is related to the charge balance in seawater:
TA = [HCO3-] + 2 [CO32-] + [B(OH)4-] + [OH-] - [H+] (5)
+ minor compounds .
Methods of Analysis
Of the carbonate system parameters, pCO2, pH, TCO2, and TA can be determined analytically. However, if any two parameters and total dissolved boron are known, all parameters (pCO2, [CO2], [HCO3-], [CO32-], pH, TCO2, and TA) can be calculated at given temperature, salinity and pressure. The pCO2 of a seawater sample refers to the pCO2 of a gas phase in equilibrium with that seawater sample. It is usually measured by equilibrating a small volume of gas with a large volume of seawater at given temperature. Then the mixing ratio of CO2(g) in the gas phase is determined either using a gas chromatograph or an infrared analyzer. Finally, the fugacity is calculated from the mixing ratio. pH is usually measured using a glass/reference electrode cell or spectrophotometrically using an indicator dye. TCO2 is usually measured by an extraction/coulometric method or a closed cell titration. A potentiometric titration is used to determine TA. TCO2 and TA are conservative quantities, that is, their concentrations measured in units of mol kg-1 are unaffected by changes in pressure or temperature, for instance, and they obey the linear mixing law. Therefore they are the preferred tracer variables in numerical models of the ocean's carbon cycle.
Ocean Data
A great number of data on the carbonate chemistry of the oceans has been obtained over the last decades from programs such as GEOSECS (Geochemical Ocean Sections Study), TTO (Transient Tracers in the Oceans), and WOCE (World Ocean Circulation Experiment). Many of these data have been made available by CDIAC (Carbon Dioxide Information Analysis Center).
Anthropogenic CO2
Since the beginning of the industrialization, the oceans have taken up about 50% of the anthropogenic CO2 produced by fossil fuel burning and cement-manufacturing (cf. greenhouse gases). The carbon dioxide dissolves in seawater, produces hydrogen ions and neutralizes carbonate ions (CO32-):
CO2 + H2O + CO32- → 2 HCO3- (6)
While the increase in surface ocean dissolved CO2 is proportional to that in the atmosphere (upon equilibration after ~1 y), the increase in TCO2 is not. This is a result of the buffer capacity of seawater. The relative change of dissolved CO2 to the relative change of TCO2 in seawater in equilibrium with atmospheric CO2 is described by the so-called Revelle factor:
R = (d[CO2]/[CO2]) / (d[TCO2]/[TCO2]) (7)
which varies roughly between 8 and 15, depending on temperature and pCO2. As a consequence, the man-made increase of TCO2 in surface seawater (ocean acidification) occurs not in a 1:1 ratio to the increase of atmospheric CO2 (the latter being mainly caused by fossil fuel burning). Rather, a doubling of pCO2 only leads to an increase of TCO2 of the order of 10%.
Marine Processes
The dominant processes changing the carbonate chemistry of a water parcel in the ocean can be described by considering changes in TCO2 and TA. Invasion of CO2 from - or release to the atmosphere increases and decreases TCO2, respectively, while TA stays constant. This leads to a rise and drop of [CO2], respectively, while the change in pH is opposite (CO2 is a weak acid). Remineralization and photosynthesis lead to the same trends, except that TA changes slightly due to nutrient release and uptake. CaCO3 precipitation decreases TCO2 and TA in a ratio 1:2, and, surprisingly, increases [CO2] although inorganic carbon is reduced. Consider the chemical reaction
Ca2+ + 2 HCO3- → CaCO3 + CO2 + H2O (8)
which qualitatively indicates that during CaCO3 precipitation CO2 is liberated. Quantitatively, however, the conclusion that [CO2] in solution is increasing by one mole per mole CaCO3 precipitated is incorrect. The correct analysis takes into account the decrease of TCO2 and TA in a ratio 1:2 and the buffer capacity of seawater. That is, the medium gets more acidic because the decrease in alkalinity outweighs that of total carbon and hence [CO2] increases. For instance, at surface ocean conditions (TCO2=2000 μmol kg-1, pH=8.2, T=15°C, S=35), [CO2] increases by only ~0.03 μmol per μmol CaCO3 precipitated.
Further Reading
- DOE. Handbook of methods for the analysis of the various parameters of the carbon dioxide system in sea water; version 2, (eds. Dickson, A.G. and Goyet, C.) ORNL/CDIAC-74, 1994.
- Millero, F. J., Thermodynamics of the carbon dioxide system in the oceans, Geochim. Cosmochim. Acta, 59, 661-677, 1995.
- Stumm, W. and J. J. Morgan, Aquatic Chemistry (3rd ed.), Wiley & Sons, New York, pp. 1022, 1996.
- Zeebe, R. E. and D. A. Wolf-Gladrow, CO2 in Seawater: Equilibrium, Kinetics, Isotopes, Elsevier Oceanography Series, 65, pp. 346, Amsterdam, 2001.