The Properties of Water
Water is the most abundant compound on Earth's surface. In nature, water exists in the liquid, solid, and gaseous states. It is in dynamic equilibrium between the liquid and gas states at 0 degrees Celsius and 1 atm of pressure. At room temperature (approximately 25 degrees Celsius), it is a tasteless, odorless, and colorless liquid. Many substances dissolve in water, and it is commonly referred to as the universal solvent.
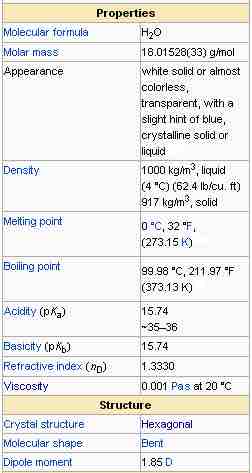
The properties of water
A table of several of the chemical and physical properties of water.
The Phases of Water
Similar to many other substances, water can take numerous forms. Its liquid phase, the most common phase of water on Earth, is the form that is generally meant by the word "water."
Solid Phase (Ice)
The solid phase of water is known as ice and commonly takes the structure of hard, amalgamated crystals, such as ice cubes, or of loosely accumulated granular crystals, such as snow. Unlike most other substances, water's solid form (ice) is less dense than its liquid form, as a result of the nature of its hexagonal packing within its crystalline structure. This lattice contains more space than when the molecules are in the liquid state.
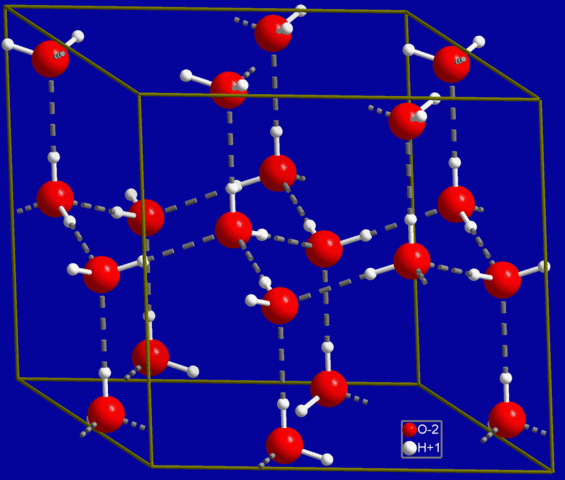
The hexagonal structure of ice
As a naturally occurring crystalline inorganic solid with an ordered structure, ice is considered to be a mineral. It possesses a regular crystalline structure based on the molecular structure of water, which consists of a single oxygen atom covalently bonded to two hydrogen atoms: H-O-H.
The fact the density of ice is less than that of liquid water's has the important consequence that ice floats.

The density of ice and water as a function of temperature
The solid form of most substances is denser than the liquid phase; therefore, a block of a given solid will generally sink in its corresponding liquid. However, a block of ice floats in liquid water because ice is less dense than liquid water. The inset shows the curve in more detail in the range of 0-10 degrees Celsius. Liquid water is most dense at 4 degrees Celsius.
Liquid Phase (Water)
Water is primarily a liquid under standard conditions (25 degrees Celsius and 1 atm of pressure). This characteristic could not be predicted by its relationship to other, gaseous hydrides of the oxygen family in the periodic table, such as hydrogen sulfide. The elements surrounding oxygen in the periodic table – nitrogen, fluorine, phosphorus, sulfur, and chlorine – all combine with hydrogen to produce gases under standard conditions. Water forms a liquid instead of a gas because oxygen is more electronegative than the surrounding elements, with the exception of fluorine. Oxygen attracts electrons much more strongly than does hydrogen, resulting in a partial positive charge on the hydrogen atoms and a partial negative charge on the oxygen atom. The presence of such a charge on each of these atoms gives a water molecule a net dipole moment.
The electrical attraction between water molecules caused by this dipole pulls individual molecules closer together, making it more difficult to separate the molecules, and therefore raising the boiling point. This type of attraction is known as hydrogen bonding. The molecules of water are constantly moving in relation to each other, and the hydrogen bonds are continually breaking and reforming at intervals briefer than 200 femtoseconds (200 x 10-15 seconds).
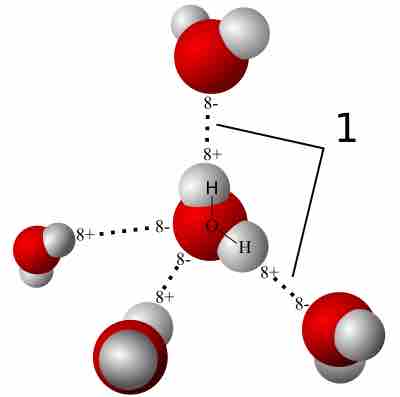
Arrangement of water molecules in the liquid phase
Water molecules align based on their polarity, forming hydrogen bonds (signified by "1").
Many of the physical and chemical properties of water (including its capacity as a solvent) are partly to the acid-base reactions it can be part of. Water can be described as an amphoteric molecule, meaning that it can react as both a Brønsted-Lowry acid or base. This can be shown in the reaction between two water molecules that produces the hydronium ion (H3O+) and a hydroxide ion (OH-):
Gas Phase (Water Vapor)
The gaseous phase of water is known as water vapor (or steam) and is characterized by a transparent cloud. Water also exists in a rare fourth state called supercritical fluid, which occurs only in extremely uninhabitable conditions. When water achieves a specific critical temperature and a specific critical pressure (647 K and 22.064 MPa), the liquid and gas phases merge into one homogeneous fluid phase that shares properties of both gas and liquid.
Phase Diagram of Water
Water freezes to form ice, ice thaws to form liquid water, and both water and ice can transform into the vapor state. Phase diagrams help describe how water changes states depending on the pressure and temperature.
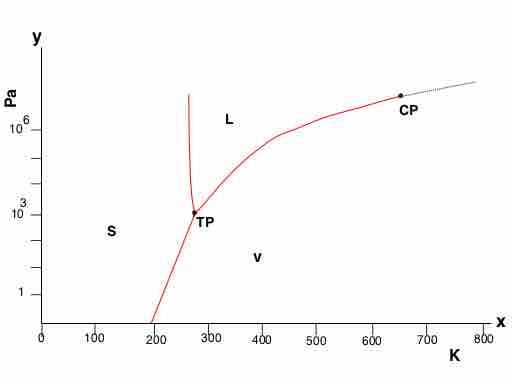
Phase diagram of water
The three phases of water – liquid, solid, and vapor – are shown in temperature-pressure space.
Note the following key points on a phase diagram:
- The critical point (CP), above which only supercritical fluids exist.
- The triple point (TP), a well-defined coordinate where the curves intersect, at which the three states of matter (solid, liquid, gas) exist at equilibrium with each other.
- Well-defined boundaries between solid and liquid, solid and gas, and liquid and gas. During the phase transition between two phases (i.e, along these boundaries), the phases are in equilibrium with each other.
The Polarity of Water
The polar nature of water is a particularly important feature that contributes to the uniqueness of this substance. The water molecule forms an angle with an oxygen atom at the vertex and hydrogen atoms at the tips. Because oxygen has a higher electronegativity than hydrogen, the side of the molecule with the oxygen atom has a partial negative charge. An object with such a charge difference is called a dipole (meaning "two poles"). The oxygen end is partially negative, and the hydrogen end is partially positive; because of this, the direction of the dipole moment points from the oxygen toward the center position between the two hydrogens. This charge difference causes water molecules to be attracted to each other (the relatively positive areas are attracted to the relatively negative areas), as well as to other polar molecules. This attraction contributes to hydrogen bonding and explains many of water's properties (including its ability to act as a solvent to many substances).
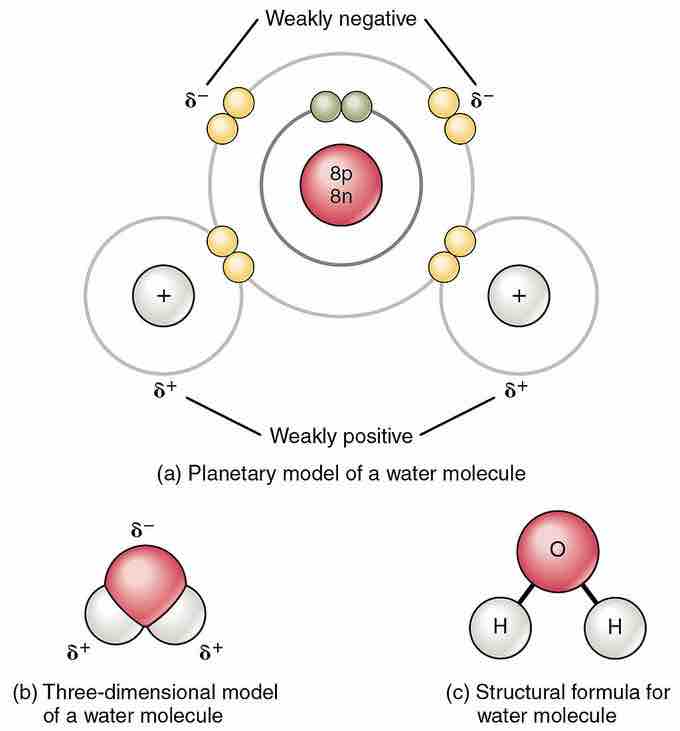
Polarity of the water molecule
Owing to the electronegativity difference between hydrogen (H) and oxygen (O) atoms, and the bent shape of the H2O molecule, a net dipole moment exists. The figure indicates the partial charges that the atoms possess.
A water molecule can form a maximum of four hydrogen bonds by accepting two hydrogen atoms and donating two hydrogen atoms. Although hydrogen bonding is a relatively weak attraction compared to the covalent bonds within the water molecule itself (intramolecular bonds), it is responsible for a number of water's physical properties. One such property is its relatively high melting and boiling points; more energy is required to break the hydrogen bonds between molecules in order to change to a higher energy phase.