Chapter 4
Aqueous Reactions
By Boundless

Unlike nonelectrolytes, electrolytes contain dissolved ions that enable them to easily conduct electricity.
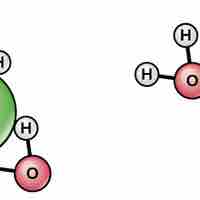
Water's polarity makes it an excellent solvent for other polar molecules and ions.

When electrodes are placed in an electrolyte solution and a voltage is applied, the electrolyte will conduct electricity.

Precipitation reactions transform ions into an insoluble salt in aqueous solution.
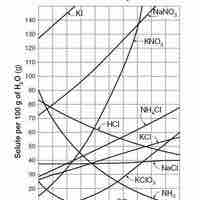
Solubility is the relative ability of a solute (solid, liquid, or gas) to dissolve into a solvent and form a solution.

Precipitation reactions can be written as molecular, ionic, or complete ionic equations.
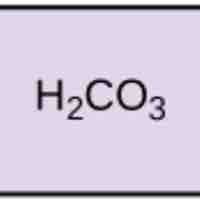
Acids dissociate into H+ and lower pH, while bases dissociate into OH- and raise pH; buffers can absorb these excess ions to maintain pH.
A Brønsted acid is any species capable of donating a proton; a Brønsted base is any capable of accepting a proton.
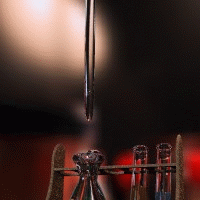
Acid-base titration can determine the concentrations of unknown acid or base solutions.
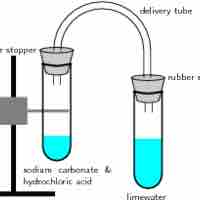
A gas evolution reaction is a chemical process that produces a gas, such as oxygen or carbon dioxide.

Oxidation state is the hypothetical charge of an atom if all of its bonds to other atoms were completely ionic.
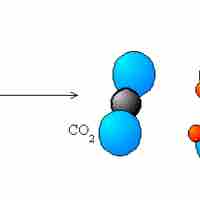
The five main types of redox reactions are combination, decomposition, displacement, combustion, and disproportionation.
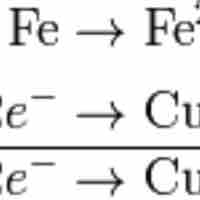
Balancing redox reactions involves splitting the reaction into two half-reactions.
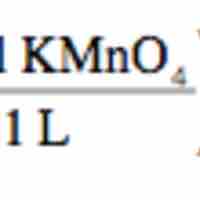
Redox titration determines the concentration of an analyte containing either an oxidizing or a reducing agent.
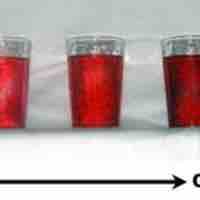
Diluting a solution involves adding additional solvent to decrease the solution's concentration.
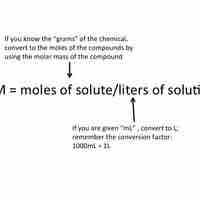
Molarity is a unit of concentration; it is equal to moles of solute divided by the total volume of the solution in liters.

Stoichiometry can be used to calculate the quantitative relationships between species in aqueous solution.