Organic chemistry is a vast field in which an infinite number of molecules can be created from a wide and ever-increasing variety of reactions. These reactions are typically much more complex than inorganic reactions, and sometimes occur in many steps.
Consider, for example, the Claisen Rearrangement:
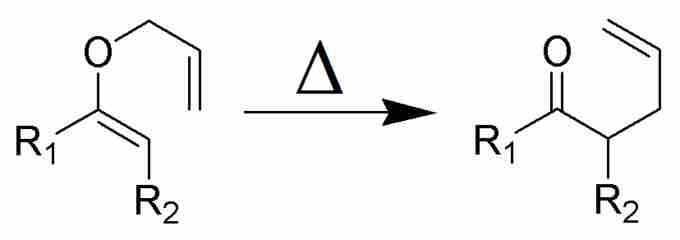
Claisen Rearrangement
When heated, alkadiene ether on the left rearranges to form the ketoalkene on the right.
What starts as an ether with two alkene units is converted to a ketone with just one alkene functionality. Chemists can analyze starting materials and products to determine structures, but to understand exactly why an organic reaction works, they must determine its mechanism. This sometimes requires experimentation with tests of reaction kinetics, but mechanisms can typically be devised on paper.
Organic reaction mechanisms are written using curved arrows that depict transfers of either nonbonding or bonding electrons to form a new bond or exist as nonbonding electrons attached to an atom. For example, consider the Diels-Alder reaction:
Without the curved arrows, it may be unclear as to how to the alkene and diene are converted to the cycloalkene. However, the arrows show that the alkene bonds all shift circularly: electrons from one bond in the diene are used to form a bond to the alkene, breaking the alkene's bond. Simultaneously, the alkene attaches to the opposite side of the diene, forcing an alkene bond to rotate:

Diels-Alder Reaction Mechanism
Note that the number of bonds plus lone pairs (there are 0 lone pairs) is conserved in this process. There are three alkene bonds in the reactants, that are broken. In their place come two new single C-C bonds and a new alkene bond.
Although the number of possible organic reactions is massive and ever-growing, fundamentally, they can be categorized into just five groups based on their mechanisms: addition, elimination, substitution, redox, and rearrangement. Each of these reaction types will be later discussed.